Abstract
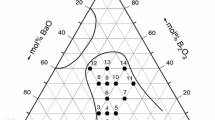
Barium aluminosilicate glasses are being investigated as matrix materials in high-temperature ceramic composites for structural applications. Kinetics of crystallization of two refractory glass compositions in the barium aluminosilicate system have been studied by differential thermal analysis (DTA), x-ray diffraction (XRD), and scanning electron microscopy (SEM). From variable heating rate DTA, the crystallization activation energies for glass compositions (wt. %) 10BaO–38Al2O3–51SiO2–1MoO3 (glass A) and 39BaO–25Al2O3–35SiO2–1MoO3 (glass B) were determined to be 553 and 558 kJ/mol, respectively. On thermal treatment, the crystalline phases in glasses A and B were identified as mullite (3Al2O3 · 2SiO2) and hexacelsian (BaO · Al2O3 · 2SiO2), respectively. Hexacelsian is a high-temperature polymorph which is metastable below 1590 °C. It undergoes structural transformation into the orthorhombic form at ∼300 °C accompanied by a large volume change which is undesirable for structural applications. A process needs to be developed where stable monoclinic celsian, rather than hexacelsian, precipitates out as the crystal phase in glass B.
Similar content being viewed by others
Change history
31 January 2011
An Erratum to this paper has been published: https://doi.org/10.1557/JMR.1990.1781
References
E. M. Levin and H. F. McMurdie, Phase Diagrams for Ceramists–1975 supplement (The American Ceramic Society, Columbus, OH, 1975), p. 220.
N. P. Bansal and R. H. Doremus, J. Thermal Anal. 29, 115 (1984).
W. A. Johnson and R. F. Mehl, Trans. Am. Inst. Elect. Eng. 135, 416 (1939).
M. Avrami, J. Chem. Phys. 7, 1103 (1939); 8, 212 (1940); 9, 177 (1941).
N. P. Bansal, R. H. Doremus, A. J. Bruce, and C. T. Moynihan, J. Am. Ceram. Soc. 66, 233 (1983).
N. P. Bansal, A. J. Bruce, R. H. Doremus, and C. T. Moynihan, J. Non-Cryst. Solids 70, 379 (1985).
N. P. Bansal, A. J. Bruce, R. H. Doremus, and C. T. Moynihan, in “Infrared Optical Materials and Fibers III,” Proc. SPIE 484, 51 (1984), Soc. Photo-Optical Instr. Engr., Bellingham, WA.
W. F. Hammetter and R. E. Loehman, J. Am. Ceram. Soc. 70, 577 (1987).
D. Bahat, J. Mater. Sci. 4, 855 (1969).
J. S. Moya Corral and A. Garcia Verduch, Trans. J. Br. Ceram. Soc. 77, 40 (1978).
D. Bahat, J. Mater. Sci. 4, 847 (1969).
S. D. Stookey, Glastech. Ber. 32, 1 (1959).
B. Vonnegut, J. Appl. Phys. 18, 593 (1947).
T. Ito, X-ray Studies on Polymorphism (Maruren Co. Ltd., Tokyo, Japan, 1956).
R. E. Newnham and H. D. Megaw, Acta Cryst. 13, 303 (1960).
D. Bahat, J. Mater. Sci. 5, 805 (1970).
M. Yoshiki and K. Matsumoto, J. Am. Ceram. Soc. 34, 283 (1951).
C. H. Drummond, W. E. Lee, N. P. Bansal, and M. J. Hyatt, paper presented at the 13th Annual Conference on Composites Materials and Structures, Restricted Sessions, Cocoa Beach, FL, Jan. 18–20, 1989; Ceram. Eng. Sc. Proc. (to be published).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bansal, N.P., Hyatt, M.J. Crystallization kinetics of BaO–Al2O3–SiO2 glasses. Journal of Materials Research 4, 1257–1265 (1989). https://doi.org/10.1557/JMR.1989.1257
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.1989.1257