ABSTRACT
The aim of this research was to investigate the physicochemical and functional properties of fresh and pasteurized chicken and duck egg albumens. The results showed that pasteurization of both chicken and duck albumens significantly decreased (p≤0.05) viscosity, but had no impact (p>0.05) on pH or free sulfhydryl groups. Chicken albumen was shown to have higher (p≤0.05) foam expansion, but lower (p≤0.05) foam stability than duck albumen. Pasteurization decreased (p≤0.05) the foam expansion of both albumens while decreasing (p≤0.05) the foam stability only of duck albumen. Investigation of the gel properties showed that duck albumen has greater hardness and lower expressible water (p≤0.05) than chicken albumen. Pasteurization increased the hardness and decreased the expressible water of both the chicken and duck albumen gels. This study suggests that the superior gel properties of duck albumen offer potential approaches to improving the quality of gel food products.
Keywords:
Egg albumen; Egg white; Chicken egg; Duck egg; Protein functionalities; Gelling; Foaming
INTRODUCTION
Eggs from chickens and ducks are two of the most consumed bird eggs in the world. Eggs are an excellent source of complete protein because they contain all of the essential amino acids (Abeyrathne et al., 2013Abeyrathne EDNS, Leel HY, Ahn DU. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents-A review. Poultry Science 2013;92:3292-3299.; Gutierrez et al., 1997Gutierrez MA, Takahashi H, Juneja LR. Nutritive evaluation of hen eggs. In: Yamamoto T, Juneja LR, Hatta H, Kim M. Hen eggs: their basic and applied science. Boca Raton: CRC Press; 1997. p.25-35.). They are considered high-cholesterol foods - 50 g of chicken and duck eggs contain as much as 200 and 450 mg of cholesterol, respectively - and public health recommendations often recommend limiting daily cholesterol levels to below 300 mg. However, recent studies suggest that consumption of a single chicken egg daily is not linked to an elevated risk of incident coronary heart disease (Virtanen et al., 2016Virtanen JK, Mursu J, Virtanen HE, Fogelholm M, Salonen JT, Koskinen TT, et al. Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein E phenotype in men: the kuopio ischaemic heart disease risk factor study. American Journal of Clinical Nutrition 2016;103:895-901.). Eggs are also used as ingredients by the food industry in a wide array of commercial products (Mehdizadeh et al., 2015Mehdizadeh SA, Minaei S, Torshizi MAK, Mohajerani E. Effect of UV irradiation, sample thickness and storage temperature on storability, bacterial activity and functional properties of liquid egg. Journal of Food Science and Technology 2015;52:4414-4422.) because of their taste, nutritional value, and unique physicochemical properties such as water-holding capacity, oil binding, emulsification, foam formation, gelation and viscosity (Wu et al., 2009Wu H, Wang Q, Ma T, Ren J. Comparative studies on the functional properties of various proteins concentrate preparations of peanut protein. Food Research International 2009;42:343-348.).
The egg albumen, also known as egg white, is compositionally similar in chickens and ducks (~88% water and 11% protein) and has many functional properties. Two of the most important functional properties of the egg albumen are the ability to produce foam and to form heat-induced gels (Wang and Wang, 2009Wang G, Wang T. Effects of yolk contamination, shearing, and heating on foaming properties of fresh egg white. Journal of Food Science 2009;74:C147-56.; Mehdizadeh et al., 2015Mehdizadeh SA, Minaei S, Torshizi MAK, Mohajerani E. Effect of UV irradiation, sample thickness and storage temperature on storability, bacterial activity and functional properties of liquid egg. Journal of Food Science and Technology 2015;52:4414-4422.). Foam formation and gelation affect both the texture and the sensory properties of the final food products. Foam formation is key for the production of meringues, bread, cookies, cakes, and several bakery products. Water-holding capacity of the egg albumen is related to its ability coagulate and link or bind with other ingredients (Mine, 1995Mine Y. Recent advances in the understanding of egg-white protein functionality. Trends in Food Science and Technology 1995;6:225-232.) in products such as ham, sausages, surimi, and noodles (Hatta et al., 1997Hatta H, Hagi T, Hirano K. Chemical and physicochemical properties of hen eggs and their application in foods. In: Yamamoto T, Juneja LR, Hatta H, Kim M. Hen eggs: their basic and applied science. Boca Raton: CRC Press; 1997. p. 117-134.). However, the efficacy of these functional properties of the albumen are affected by intrinsic and extrinsic factors such as protein composition and concentration, heating temperature, heating time, pH, ionic strength, and the presence of other additives; therefore, different types of egg may exhibit different performance. The functional properties of the egg albumen are well-studied (Cunningham, 1995Cunningham FE. Egg-product pasteurization. In: Stadelman WJ, Cotterill OJ. Egg science and tTechnology. 4th ed. Binghampton: Hawthorn Press; 1995. p.289-321.; Hatta et al., 1997; Plancken et al., 2005Plancken V, Van LA, Hendrickx ME. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. Journal of Agricultural and Food Chemistry 2005;53:5726-5733.; Mine, 1995; Mehdizadeh et al., 2015); however, information about duck albumen functionality is much less common, especially in comparison with chicken albumen.
Pasteurization is required to destroy pathogens in the albumen but causes denaturation and aggregation of some egg proteins, resulting in impaired protein functionalities (Cunningham, 1995Cunningham FE. Egg-product pasteurization. In: Stadelman WJ, Cotterill OJ. Egg science and tTechnology. 4th ed. Binghampton: Hawthorn Press; 1995. p.289-321.). Heating the albumen to the US standard pasteurization conditions of 55.6°C for 6.2 min or 56.7°C for 2 min is required to reduce the pathogenic bacterium Salmonella (Code of Federal Regulations, 2010). However, thermal processing causes some of proteins in the albumen to become denatured, affecting their function and making the albumen unsuitable as an ingredient in certain food products (Hou et al., 1996Hou H, Singh RK, Muriana PM, Stadelman WJ. Pasteurization of intact shell eggs. Food Microbiology 1996;13:93-101.). A literature search revealed no previous studies that have investigated the functionality of pasteurized versus fresh albumen from duck and chicken eggs.
Chicken egg albumen is a common industrial food ingredient. Duck yolk is a common ingredient in Asian dishes and in the Thai dessert industry; however, duck albumen is a by-product and not currently used as a food ingredient in the food industry. The objective of this research was to compare the foaming and gelation properties of the albumen from fresh and pasteurized chicken and duck eggs. The information obtained in this study could provide guidelines for the utilization of duck albumen in the food industry.
MATERIALS AND METHODS
Materials
Four hundred fresh chicken eggs of Hisex Brown® hens between 31-32 wk of age and four hundred fresh duck eggs of Anas platyrhucus layers between 30-31 wk of age were collected from farms in Pathum Thani, Thailand. The eggs were kept at 28-32 °C (normal egg storage conditions in Thailand) for less than three days.
Sodium dihydrogenphosphate and tri-sodium phosphate were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). DTNB (5, 5’-dithiobis (2-nitrobenzoic acid)) and ANS- (1, 8-anilinonapthlensulfonate) were purchased from Sigma Aldrich (St. Louis, MO). Analytical grade hydrochloric acid and sodium hydroxide were purchased from Carlo Erba Reagents (Carlo Erba, Milan, Italy). All chemicals were used directly from the sample container without further purification. Distilled and de-ionized water was used for preparation of all buffer solutions. The pH of all buffer solutions used in this study was measured using a pH meter (SevenEasyTM pH Meter S20, Mettler-Toledo, Schwerzenbach, Switzerland).
Preparation of the Egg Albumen
Forty eggs were randomly sampled, cleaned, and manually cracked, after which the albumen was separated and collected. The albumen was placed in an ice bath and maintained at 0°C for 30 min. The albumen was transferred into a 50 mL syringe then injected through a 60-mesh stainless sieve screen to remove large particles. The screening was repeated three times within 20 min to ensure the homogeneity of the sample. The albumen was kept in the ice bath until further use. Three sets of chicken or duck egg albumens were separately prepared, and the albumens were separately used for further experiments.
Preparation of Pasteurized Egg Albumen
Two hundred grams of the albumen were transferred to a 400-mL beaker, and then equilibrated to 30°C using a water bath. After the temperature of the sample reached 30°C, the beaker containing the albumen was transferred to a 66°C water bath. The albumen was equilibrated with stirring at 1300 rpm (IKA, C-MAG MS 7, Germany). After the temperature reached 56.5°C, the beaker was transferred to a 56.5 ± 1.0°C water bath. The albumen was further incubated with stirring at 1300 rpm for 3.5 min then put into an ice bath with stirring at the same speed for 10 min.
Proximate Analysis
Proximate analysis such as moisture, ash, crude fat, fiber and protein contents were determined using the methods of AOAC (2000AOAC. Official methods of analysis of AOAC International. 17th ed. Washington: Association of Official Analytical Chemists; 2000.). The carbohydrate content was calculated by subtraction from the other contents.
Determination of Haugh Units
The Haugh unit was used to determine egg freshness, as described by Haugh (1937Haugh RR. A new method for determining the quality of an egg. US Poultry Magazine 1937;39:27-49.). Forty eggs were sampled to measure the Haugh unit, which value was calculated as follows:
Where:
h = Observed albumen thickness (mm)
w = Weight of the entire egg (g)
Determination of Physical Properties
Albumen pH, density, viscosity and solid content were determined by pH meter (SevenEasyTM pH Meter S20, Mettler-Toledo, Schwerzenbach, Switzerland), pycnometer (Wilmad-LabGlass, USA), Brookfield viscometer equipped with RV1 spindle (Eng Labs DE33009, USA) and moisture balance (Satorius MA30, Germany), respectively. For density and viscosity, samples were performed at 25°C.
Determination of Exposed Free-Sulfhydryl Groups of Protein
The influence of pasteurization on the exposed free sulfhydryl (SH) groups of the albumen was measured using Ellman’s reagent [5, 5’-dithiobis (2-nitrobenzoic acid)] or DTNB, as described by Beveridge and others (1974Beveridge T, Toma SJ, Nakai S. Determination of SH- and SS- groups in some food proteins using Ellman's Reagent. Journal of Food Science 1974;39:49-51.) with some modification. Briefly, albumen was equilibrated at room temperature for 1 h. One milliliter of albumen was suspended in 9 mL of distilled water then vortexed for 10 s. Five hundred microliters of the mixture were diluted with 500 µl of standard buffer pH 8.0 (containing 86 mM trisaminomethane (TRIS), 90 mM glycine and 4 mM ethylenediaminetetraacetic acid (EDTA)). Ten microliters of Ellman’s reagent solution (4 mg of DTNB/mL of standard buffer) were added to the mixed solution then allowed to incubate at room temperature for 1 h. The mixture was centrifuged at 13,000×g, 20°C for 20 min. The supernatant was collected to determine the exposed free sulfhydryl group content. Absorbance was recorded at 412 nm using a UV-Vis spectrophotometer (Helios Alpha, Thermo Electron Corporation, England). The standard buffer was used as blank. The free sulfhydryl groups (mmole SH/g protein) of each sample were calculated using a molar extinction coefficient of 1.36x104 M-1 cm-1. All measurements were done on three freshly prepared samples.
Determination of Surface Hydrophobicity of Protein
The surface hydrophobicity (S0) of the chicken and duck albumen was determined using 1, 8-anilinonapthalenesulfonate (ANS-) as described by Haskard and Li-Chan (1998Haskard CA, Li-Chan ECY. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-) fluorescent probes. Journal of Agricultural and Food Chemistry 1998;46:2671-2677.). ANS- stock solution (8 mM) was prepared by dissolving ANS- in 10 mM of phosphate buffer (pH 7.0). The protein content of albumen was determined by Lowry’s method using bovine serum albumin (BSA) as a standard (Lowry et al., 1951Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 1951;193:265-275.). Egg white protein suspensions of 0.20-0.50 mg protein/mL were prepared in 10 mM of phosphate buffer pH 7.0. Four milliliters of diluted protein suspension were mixed with 20 µl of ANS- stock solution and vortexed for 5 s. The fluorescence intensity (FI) of the mixtures was measured at an excitation wavelength at 370 nm and emission wavelength at 470 nm using a microplate reader (Infinite M200 PRO, TECAN Austria GmbH, Austria). The FI of each sample was calculated by subtracting the FI of the buffer. The S0 was the initial slope of the subtracted FI versus the protein concentration calculated by linear regression analysis.
Determination of Molecular Weight of Protein
The molecular weight of the protein samples was determined as described by Laemmli (1970Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-685.). Briefly, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a discontinuous buffer system with and without b-mercaptoethanol using 12.5% separating gel and 4% stacking gel. Protein samples in loading buffer (containing 0.0625 M TRIS-HCl, 10% glycerin, 2% SDS, 0.0025% bromophenol blue, and with or without 5% b-mercaptoethanol) were loaded into the gel (10 µg of protein/well). Gel electrophoresis was performed at 20 mA for 90 min. Gel was stained with Coomassie Brilliant Blue G-250 and destained in a destaining solution (30% methanol, 10% acetic acid and 60% distilled water) prior to gel imaging. The protein content in the sample was determined using the method described in the previous section for proximate analysis.
Determination of Foaming Properties
The foaming properties of the chicken and duck albumens as well as the effect of pasteurization on the foaming properties were analyzed as described by Uzun and others (2012Uzun H, Ibanoglu E, Catal H, Ibanoglu S. Effects of ozone on functional properties of proteins. Food Chemistry 2012;134:647-654.) with some modifications. The albumen was equilibrated to room temperature for 1 h. One hundred milliliters of albumen were transferred into a stainless steel bowl (Seagull, Thai Stainless Steel Co., Ltd., Thailand) 21 cm in diameter and 8.5 cm in height, then whipped with a hand blender (HM-009 OTTO, Otto King Glass Co., Ltd., Thailand) at speed 5 (speed 1-slowest and speed 5-fastest) for 3 min. The height of the foam was measured directly using Vernier caliper at five different locations of the foam and the average height was used to calculate the volume of foam. The liquid drainage of the foam at various times (0-120 min) was collected and weighed. Foam expansion and foam stability as a percentage of drainage were calculated as follow:
Determination of Gel Hardness
The hardness of the gels and the influence of pasteurization on the hardness of the gels were studied using a method adapted from Harkouss and others (2015). Albumen was equilibrated to room temperature. Twenty milliliter of the albumen was poured into 2.5 cm diameter plastic casing. The casings were sealed at both ends then boiled for 15 min in a water bath (Boiling-Sterilizer, Applied Medic Ltd., Thailand). Samples were then immediately cooled in an ice bath for 5 min and stored at 4°C overnight. Gel samples were equilibrated to room temperature (about 25°C) for 1 h and removed from the plastic casing then cut into 1.5 × 1.5 × 2.0 cm3 (width × length × height) bricks. Texture profile analysis (TPA) of the protein gels was performed using a texture analyzer (TA XT-2i Texture Analyzer, Stable Micro Systems, England) equipped with a 50 mm diameter aluminum cylinder probe (P50) and a 20 kg load cell. Measurements were performed at a crosshead speed of 5.0 mm/s until a maximum Cauchy strain of 0.5 was reached (Woodward & Cotterill, 1986Woodward SA, Cotterill OJ. Texture and microstructure of heat-formed egg white gels. Journal of Food Science 1986;51:333-339.). Only gel hardness is reported in this paper.
Determination of Expressible Moisture
The percentage of expressible water from the egg white gel was determined using a procedure adapted from Funami and others (1998Funami T, Yada H, Nakao Y. Thermal and rheological properties of curdlan gel in minced pork gel. Food Hydrocolloids 1998;12:55-64.). The expressible water was calculated from the moisture loss after compression. Egg white gels were cut into 1.5 × 1.5 × 2.0 cm3 (width × length × height) bricks, placed between double layers of filter paper (Whatman No. 4) and then compressed using a texture analyzer at a crosshead speed of 3 mm/s to 70% strain for 60 s with a cylindrical aluminum probe (50 mm diameter). One gram of samples both before and after compression was sampled to measure the moisture content using a moisture analyzer (MA 30, Sartorius, Germany). The expressible moisture content was calculated from the following equation:
Determination of Egg White Gel Color
The color of the egg white gel was determined using a Chroma meter (CR-300, Minolta Co., Ltd., Japan) from the mean value of three measurements. Egg white gels were cut into 1.5 × 1.5 × 2.0 cm3 (width × length × height) bricks. The color was reported in the CIE Lab color system. The Chroma meter was calibrated using a Minolta calibration plate.
Statistical Analysis
Experiments were done in triplicate using freshly prepared samples. Statistical analysis was performed using one-way analysis of variance (ANOVA) (Cochran and Cox, 1992Cochran WG, Cox GM. Experimental designs, 2nd ed. New York: Wiley; 1992.) using SPSS version 11.5 (SPSS Inc., Chicago, USA). Mean separation were achieved using Duncan’s multiple range test at a 95% confidence level.
RESULTS AND DISCUSSION
Physicochemical Properties of Fresh Chicken and Duck Albumens
Since freshness is an important parameter related to protein function in egg, Haugh unit (HU) was determined for both chicken and duck eggs (Haugh, 1937Haugh RR. A new method for determining the quality of an egg. US Poultry Magazine 1937;39:27-49.). Because HU of duck eggs may be affected by the fact that duck eggs are commonly bigger and can have a different proportion of albumen, HU in this investigation was only used to control and report the freshness of each egg: the higher the number, the better the quality of the egg. The average HU values of chicken and duck eggs used in this study were 75.31 ± 7.13 and 66.28 ± 3.63, respectively. HU values of 72 or higher for chicken eggs are used by the USDA to indicate AA quality grade, indicating that the egg is fresh and the albumen is sufficiently thick. However, there is no standard for duck eggs. In this experiment, duck eggs had lower HU value than chicken eggs, even though both chicken and duck eggs used were stored for the same duration and under the same conditions.
The results from the proximate analysis are shown in Table 1. No significant difference between chicken and duck albumens was found for fat, ash, or fiber contents. The chicken albumen had a significantly higher (p≤0.05) moisture content than the duck albumen, but lower (p≤0.05) carbohydrate and protein contents. The protein content was 12.15 ± 0.08% for duck albumen and 10.03 ± 0.08% for chicken albumen. A similar result in chicken albumen was reported by Alamprese et al. (2012Alamprese C, Casiraghi E, Rossi M. Foaming, gelling and rheological properties of egg albumen as affected by the housing system and the age of laying hens. International Journal of Food Science and Technology 2012;47:1411-1420.), who found that the protein content of chicken albumen was 9.9-11.3%.
Table 2 shows the physical properties of chicken and duck albumen. The density and pH of the chicken and duck albumens were not significantly different (p>0.05). The viscosity of the duck albumen was significantly lower (p≤0.05) than that of the chicken albumen, while the solid content of the duck albumen significantly higher (p≤0.05) than that of the chicken albumen. Pasteurization significantly decreased (p≤0.05) the viscosity of both. The viscosity decreased from 7.18 ± 0.08 to 3.87 ± 0.05 cP in the chicken albumen and from 6.25 ± 0.05 to 5.70 ± 0.09 cP in the duck albumen. Heat from the pasteurization may denature some proteins in the albumen, leading to protein aggregation. For example, denaturation of ovotransferrin, the most heat sensitive protein in the albumen, occurs between 53°C and 65°C (Mine, 1995Mine Y. Recent advances in the understanding of egg-white protein functionality. Trends in Food Science and Technology 1995;6:225-232.; Lomakina & Mikova, 2006Lomakina K, Mikova K. A study of the factors affecting the foaming properties of egg white-A review. Czech Journal of Food Science 2006;24:110-118.). Thus, heat treatment could change the ovotransferrin from native to denatured form, altering the viscosity of the albumen. Nicoud and coworkers (2015Nicoud L, Lattuada M, Yates A, Morbideli M. Impact of aggregate formation on the viscosity of protein solutions. Soft Matter 2015;11(27):5513-5522.) found that in some proteins the viscosity of an aggregate sample was lower than that of a monomeric sample of a similarly-occupied volume fraction due to the polydispersity of the aggregate distribution. In addition, the shear forces used in heat treatment may disrupt high molecular weight complexes such as ovomucin in thick albumen (Lang & Rha, 1982Lang ER, Rha C. Apparent shear viscosity of native egg white. International Journal of Food Science and Technology 1982;17:595-606.). This could be another reason that pasteurization caused the viscosity to decrease.
SDS-PAGE Patterns of Chicken and Duck Albumen Proteins
The molecular weight of chicken and duck albumen proteins was determined by SDS-PAGE, shown in Figure 1. The results show that the SDS-PAGE pattern of duck albumen was different from that of chicken both in the presence and absence of a reducing agent. These results indicate that the protein composition of the duck and chicken albumens was different. For SDS-PAGE without the reducing agent, duck albumen had lower lysozyme (Mw ~ 14 kDa (Mine, 1995Mine Y. Recent advances in the understanding of egg-white protein functionality. Trends in Food Science and Technology 1995;6:225-232.)) and ovotransferrin (Mw ~76 kDa (Mine, 1995)) contents, but higher ovomucin (Mw ~ 254 kDa for a carbohydrate-poor a-ovomucin and Mw ~ 400-610 kDa for a carbohydrate-rich b-ovomucin (Offengenden et al., 2011Offengenden M, Fentabil MA, Wu J. N-glycosylation of ovomucin from hen egg white. Glycoconjugate Journal 2011;28:113-123.)) contents. It should be noted that the ovomucoid (Mw ~ 28 kDa) (Mine, 1995) of the chicken albumen appeared at 30 to 40 kDa in SDS-PAGE profiles, possibly because the band could not be separated from the ovalbumin band (a major albumen protein and Mw ~ 45 kDa) (Mine, 1995). In addition, a related band with higher molecular weight also appeared on the duck albumen profile. Similar results were found in SDS-PAGE with a reducing agent in both chicken and duck albumens. The results also indicated that a heat process in which the temperature was raised to 56.5°C then held for 3.5 min did not lead to detectable levels of protein aggregation on the SDS-PAGE patterns of chicken or duck albumen, as shown in Figure 1.
Influence of pasteurization on SDS-PAGE patterns with and without reducing agent of chicken and duck egg albumen.
Free Sulfhydryl Content in Chicken and Duck Albumen Proteins
The purpose of this experiment was to compare the exposed free sulfhydryl groups of the two egg albumens and to study the influence of pasteurization on the free sulfhydryl content. Table 2 shows the free sulfhydryl group content of both the pasteurized and non-pasteurized albumens. The free sulfhydryl groups of the non-pasteurized duck albumen were significantly higher (p≤0.05) than those of the non-pasteurized chicken albumen. The free sulfhydryl groups in non-pasteurized duck and chicken albumens were 2.63 ± 0.51 and 0.48 ± 0.00 µmol SH/g protein, respectively. Pasteurization had no impact (p>0.05) on the free sulfhydryl content of either albumens, as shown in Table 2. This result suggests that there was no heat-induced exposure of buried SH groups by the pasteurization parameters employed in this study and differs from whey protein, where research suggests that heat treatment could change the free sulfhydryl groups of protein to disulfide bonds and affect whey functional properties (Havea et al., 2004Havea P, Carr AJ, Creamer LK. The roles of disulphide and non-covalent bonding in the functional properties of heat-induced whey protein gels. Journal of Dairy Research 2004;71:330-339.).
Surface Hydrophobicity (S 0 ) of Chicken and Duck Albumen Proteins
Non-pasteurized duck and chicken albumens had similar S0 values, as shown in Table. 2. The S0 of non-pasteurized duck and chicken egg albumen was 3.85 ± 0.03 x 104 and 3.97 ± 0.08 x 104, respectively. Pasteurization had an impact on the S0 of both albumens. Pasteurization significantly increased (p≤0.05) the S0 of the duck albumen by 62% and of the chicken albumen by 105%. It may be surmised that chicken albumen proteins are more heat-sensitive than those of duck albumen.
Foam Capacity and Foam Stability
Foam expansion is an important functional property of the egg albumen. Table 3 shows that the chicken albumen had a significantly higher (p≤0.05) foam expansion than the duck albumen. Foam expansion of non-pasteurized chicken albumen was 811 ± 56% and that of the duck albumen was 626 ± 75%. Foam is a colloidal system with a solid continuous phase trapping air. Foam stability depends on the ability of a protein to adsorb and form a protective membrane at liquid-air interface. This result suggests that chicken albumen forms more stable liquid-air film than that of ducks. Pasteurization lowered the foam expansion of both chicken and duck albumens, indicating that pasteurization decreased membrane strength at the liquid-air interface. These results could be explained by excessive protein denaturation caused by the heat treatment, reducing the foam formation ability of the protein. Denatured proteins may be less able to form the two-phase colloidal system and trap air than partially denatured proteins. Pasteurization had a stronger impact on duck albumen, decreasing foam expansion by nearly 41%, compared with approximately 18% for the chicken albumen.
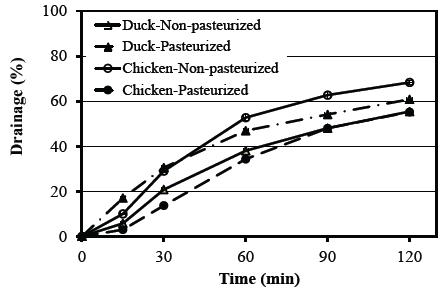
Figure 2 shows the relationship between foam drainage and incubation time in non-pasteurized and pasteurized albumens. The weight of the drainage from albumen increased over time. It should be noted that lower drainage indicates higher foam stability. Non-pasteurized duck albumen had lower (p≤0.05) drainage than non-pasteurized chicken albumen, giving it higher foam stability (p≤0.05). This suggests that the membrane produced by the duck albumen was stronger than that of the chicken albumen. Pasteurization had an impact on the drainage of both albumens. While pasteurization significantly (p≤0.05) increased the foam stability of the chicken albumen, the opposite was found for the duck albumen. These results could be explained by the strength of the film that trapped and separated the air bubbles after heat treatment, and suggest that it increased in the chicken albumen, but decreased in the duck albumen. Shown in Table 3, the drainage recorded after 1 h of foam storage indicated foam stability in the following order: pasteurized chicken albumen > non-pasteurized duck albumen > pasteurized duck albumen > non-pasteurized chicken albumen. Pasteurization changed the drainage of duck albumen from 38.0 ± 0.6 to 46.9 ± 0.5% and chicken albumen from 52.7 ± 1.2 to 34.3 ± 1.5%.
Gel Properties and Expressible Water
Gel formation is another important functional property of the egg white protein. Table 4 shows the hardness of the gels prepared from non-pasteurized and pasteurized duck and chicken albumens. The non-pasteurized duck albumen gel had significantly (p≤0.05) higher gel hardness than the non-pasteurized chicken albumen gel. These results may be attributed to the free sulfhydryl and protein contents of the albumens. Our results showed that the free sulfhydryl content of duck albumen was higher than that of chicken albumen both with and without pasteurization (Table 2). Proteins with free sulfhydryl groups form gels with higher gel hardness than proteins without free sulfhydryl groups as these free sulfhydryl groups are key in heat-induced gelation and the determination of gel strength (Zayas, 1997Zayas JF. Gelling properties of proteins. In: Zayas JR, editor. Functionality of proteins in food. Berlin: Springer; 1997. p.310-366.). During heat treatment, the free sulfhydryl groups form covalent disulfide bonds resulting in a protein gel with high hardness. In this study, pasteurization significantly increased (p≤0.05) gel hardness of the duck albumen but had no impact (p>0.05) on the gel hardness of chicken albumen, as shown in Table 4. Pasteurization increased gel hardness of the duck albumen from 19.20 ± 0.88 to 23.60 ± 0.41 N.
Table 4 shows that the duck albumen gel contained significantly lower (p≤0.05) expressible water than the chicken albumen gel. The expressible water of non-pasteurized duck and chicken albumen gels were 5.49 ± 0.43 and 6.43 ± 0.49%, respectively. These results suggest that the duck albumen gel trapped water inside the 3-dimensional gel structure more strongly than the chicken albumen gel. Pasteurization significantly decreased (p≤0.05) expressible water in both gels, by 5.49 ± 0.43 to 4.05 ± 0.09% in ducks and 6.43 ± 0.49 to 5.64 ± 0.12% in chickens. These results suggest that pasteurized albumen gel trapped water better than non-pasteurized albumen gel.
Table 4 shows the CIE Lab color of the heat-induced gels prepared from both non-pasteurized and pasteurized duck and chicken albumens. Compared with chicken gels, duck gels were significantly darker (lower L*), less green (lower -a*), slightly blue (-b*) than chicken gels, which were slightly yellow (+b*). Pasteurization had no impact on the CIE Lab attributes shown in Table 4.
SUMMARY
While side-by-side comparisons of chicken and duck meat have been made, here we report the first comparison of the functional properties of chicken and duck albumen. Overall, duck albumen contains more protein than chicken albumen, probably due to ovomucin. Duck albumen is less viscous than chicken albumen. Duck albumen has more exposed sulfhydryl groups, but its surface hydrophobicity is not different from chicken albumen. Duck albumen foams exhibited less expansion, but higher foam stability than chicken albumen foams. Duck albumen gels were harder and exhibited less expressible water than chicken albumen gels, and were darker.
It is not known how the sensory properties of egg albumen from duck differs from chicken, but culinary applications for gels and foams from duck albumen could replace chicken albumen for applications, where firmness and syneresis control would be desirable such as egg tofu, and egg loaf. There are many other food and non-food related industrial applications typically reserved for chicken albumen that may be filled by duck albumen.
CONCLUSIONS
Overall, our results demonstrated differences in the physicochemical and protein functionalities of duck and chicken albumen (as shown in Figure 3), especially foaming and gelling properties. Chicken albumen was shown to exhibit superior foam properties relative to duck albumen, whereas duck albumen had superior gel properties, including lower expressible water. These findings could be used to improve the quality of foaming and gelling food products. Our research also suggests potential uses for the duck albumen that is produced as a by-product in the Thai dessert industry and many Asian cuisines, which is currently sometimes discarded. This offers both economic and environmental benefits.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the financial support partially provided by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission.
REFERENCES
- Abeyrathne EDNS, Leel HY, Ahn DU. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents-A review. Poultry Science 2013;92:3292-3299.
- Alamprese C, Casiraghi E, Rossi M. Foaming, gelling and rheological properties of egg albumen as affected by the housing system and the age of laying hens. International Journal of Food Science and Technology 2012;47:1411-1420.
- AOAC. Official methods of analysis of AOAC International. 17th ed. Washington: Association of Official Analytical Chemists; 2000.
- Beveridge T, Toma SJ, Nakai S. Determination of SH- and SS- groups in some food proteins using Ellman's Reagent. Journal of Food Science 1974;39:49-51.
- Cochran WG, Cox GM. Experimental designs, 2nd ed. New York: Wiley; 1992.
- Code of Federal Regulations. Title 9-Animals and animal products [section 590.570]. pasteurization of liquid eggs [cited 2014 Mar 7]. 2010. Available from: http://www.gpo.gov/fdsys/pkg/CFR-2010-title9-vol2/pdf/CFR-2010-title9-vol2-sec590-570.pdf
» http://www.gpo.gov/fdsys/pkg/CFR-2010-title9-vol2/pdf/CFR-2010-title9-vol2-sec590-570.pdf - Cunningham FE. Egg-product pasteurization. In: Stadelman WJ, Cotterill OJ. Egg science and tTechnology. 4th ed. Binghampton: Hawthorn Press; 1995. p.289-321.
- Funami T, Yada H, Nakao Y. Thermal and rheological properties of curdlan gel in minced pork gel. Food Hydrocolloids 1998;12:55-64.
- Gutierrez MA, Takahashi H, Juneja LR. Nutritive evaluation of hen eggs. In: Yamamoto T, Juneja LR, Hatta H, Kim M. Hen eggs: their basic and applied science. Boca Raton: CRC Press; 1997. p.25-35.
- Harkouss R, Astruc T, Lebert A, Gatellier P, Loison O, Safa H, et al. Quantitative study of the relationships among proteolysis, lipid oxidation, structure and texture throughout the dry-cured ham process. Food Chemistry 2015;166:522-530.
- Haskard CA, Li-Chan ECY. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-) fluorescent probes. Journal of Agricultural and Food Chemistry 1998;46:2671-2677.
- Hatta H, Hagi T, Hirano K. Chemical and physicochemical properties of hen eggs and their application in foods. In: Yamamoto T, Juneja LR, Hatta H, Kim M. Hen eggs: their basic and applied science. Boca Raton: CRC Press; 1997. p. 117-134.
- Haugh RR. A new method for determining the quality of an egg. US Poultry Magazine 1937;39:27-49.
- Havea P, Carr AJ, Creamer LK. The roles of disulphide and non-covalent bonding in the functional properties of heat-induced whey protein gels. Journal of Dairy Research 2004;71:330-339.
- Hou H, Singh RK, Muriana PM, Stadelman WJ. Pasteurization of intact shell eggs. Food Microbiology 1996;13:93-101.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-685.
- Lang ER, Rha C. Apparent shear viscosity of native egg white. International Journal of Food Science and Technology 1982;17:595-606.
- Lomakina K, Mikova K. A study of the factors affecting the foaming properties of egg white-A review. Czech Journal of Food Science 2006;24:110-118.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 1951;193:265-275.
- Mehdizadeh SA, Minaei S, Torshizi MAK, Mohajerani E. Effect of UV irradiation, sample thickness and storage temperature on storability, bacterial activity and functional properties of liquid egg. Journal of Food Science and Technology 2015;52:4414-4422.
- Mine Y. Recent advances in the understanding of egg-white protein functionality. Trends in Food Science and Technology 1995;6:225-232.
- Nicoud L, Lattuada M, Yates A, Morbideli M. Impact of aggregate formation on the viscosity of protein solutions. Soft Matter 2015;11(27):5513-5522.
- Offengenden M, Fentabil MA, Wu J. N-glycosylation of ovomucin from hen egg white. Glycoconjugate Journal 2011;28:113-123.
- Plancken V, Van LA, Hendrickx ME. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. Journal of Agricultural and Food Chemistry 2005;53:5726-5733.
- Uzun H, Ibanoglu E, Catal H, Ibanoglu S. Effects of ozone on functional properties of proteins. Food Chemistry 2012;134:647-654.
- Virtanen JK, Mursu J, Virtanen HE, Fogelholm M, Salonen JT, Koskinen TT, et al. Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein E phenotype in men: the kuopio ischaemic heart disease risk factor study. American Journal of Clinical Nutrition 2016;103:895-901.
- Wang G, Wang T. Effects of yolk contamination, shearing, and heating on foaming properties of fresh egg white. Journal of Food Science 2009;74:C147-56.
- Woodward SA, Cotterill OJ. Texture and microstructure of heat-formed egg white gels. Journal of Food Science 1986;51:333-339.
- Wu H, Wang Q, Ma T, Ren J. Comparative studies on the functional properties of various proteins concentrate preparations of peanut protein. Food Research International 2009;42:343-348.
- Zayas JF. Gelling properties of proteins. In: Zayas JR, editor. Functionality of proteins in food. Berlin: Springer; 1997. p.310-366.
Publication Dates
-
Publication in this collection
2019
History
-
Received
05 Dec 2017 -
Accepted
17 Nov 2018