Abstract
Important properties in cementitious materials, such as concrete, are related to the presence of additives that influence the rigidity and the physical and chemical resistances. For the evaluation of the additive effectiveness, known as pozzolanic activity, a simple procedure, the Chapelle test, is commonly used, and it essentially consists in a reaction between the additive with calcium oxide in aqueous medium. However, such procedure presents limitations in terms of processing time and lack of information regarding the reactions kinetics. In this sense, a simple method based on the kinetic and thermodynamic principles of chemical reactions is proposed, which can be performed using conventional electronic pH sensors. The study provides an alternative methodology with many advantages over the traditional procedure, such as energy and time-savings, more robustness and more confidence. In this paper, three types of silica nanoparticles that can be used as low-cost additives were characterized in relation to their morphology and crystallinity by XRD and SEM, the particles average diameters were obtained and the particles were used for studying the chemical process that takes place during the Chapelle test. Results and the semi-empirical analysis provided strong evidence that the process is an acid-base 1:1 reaction and it was verified that the mean reaction times varied from 64 to 195 min. It is a remarkable result, since the proposed analysis can be performed with simple, fast and low-cost instrumentation and needs only a worksheet software, whereas the Chapelle test takes 16 hours and provides no dynamic information. Besides the limitation that the methodology is not able to quantify and to elucidate the effects of the specific surface area of the particles, which needs a complete BET study, the research provides a significant contribution for the understanding of the pozzolanic process, of great importance in both concrete and ceramic research.
Keywords:
Chapelle test; pozzolanic reaction; concrete; silica; quartz
1. Introduction
The advances in concrete technology rely on the use of mineral admixtures, such as silica fume, in order to produce high-performance products11 Poon CS, Lam L, Kou SC, Wong YL, Wong R. Rate of pozzolanic reaction of metakaolin in high-performance cement pastes. Cement and Concrete Research. 2001;31(9):1301-1306.. The utilization of those materials enhances the concrete properties, including durability, resistance to chloride corrosion, freezing, thawing, and deicing salting scale22 Caldarone MA, Gruber KA, Burg RG. High Reactivity Metakaolin (HRM): A New Generation Mineral Admixture for High Performance Concrete. Concrete International. 1994;16(11):37-40.,33 Zhang MH, Malhotra VM. Characteristics of a thermally activated alumino-silicate pozzolanic material and its use in concrete. Cement and Concrete Research. 1995;25(8):1713-1725..
Additions constituted of siliceous or aluminous materials in finely divided form and generally in the presence of water are usually referred as "Pozzolana"44 Perraki T, Kakali G, Kontoleon F. The effect of natural zeolites on the early hydration of Portland cement. Microporous and Mesoporous Materials. 2003;61(1-3):205-212.,55 Sabir BB, Wild S, Bai J. Metakaolin and calcined clays as pozzolans for concrete: a review. Cement and Concrete Composites. 2001;23(6):441-454.. These materials react chemically with calcium hydroxide and produce compounds with cementitious properties44 Perraki T, Kakali G, Kontoleon F. The effect of natural zeolites on the early hydration of Portland cement. Microporous and Mesoporous Materials. 2003;61(1-3):205-212.,55 Sabir BB, Wild S, Bai J. Metakaolin and calcined clays as pozzolans for concrete: a review. Cement and Concrete Composites. 2001;23(6):441-454..
The effectiveness of these additions is termed pozzolanic activity, being commonly evaluated using the Chapelle test44 Perraki T, Kakali G, Kontoleon F. The effect of natural zeolites on the early hydration of Portland cement. Microporous and Mesoporous Materials. 2003;61(1-3):205-212.,66 Kakalia G, Perraki T, Tsivilis S, Badogiannis E. Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Applied Clay Science. 2001;20(1-2):73-80., a simple procedure in which 1 g of the analyzed sample is added to 200 mL of deionized water and 1 g of calcium oxide. The mixture is boiled for 16 h, and then it is filtered and titrated with a 1 N chloride acid solution, with phenolphthalein indicator, in order to evaluate the amount of calcium oxide that has not taken part in the reaction55 Sabir BB, Wild S, Bai J. Metakaolin and calcined clays as pozzolans for concrete: a review. Cement and Concrete Composites. 2001;23(6):441-454..
The Chapelle test is relatively simple, but it does not provide information about the dynamic behavior of the chemical process. A first problem that can be pointed out is that a 16 h time is sufficiently long for occurring parallel reactions, such as the acid-base neutralization reaction of the calcium oxide by the carbon dioxide present in the air77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980., giving a false interpretation to the test result.
The test is also not able to quantify common effects in chemical reactions that can change the observed rate88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , like agitation power, solid-liquid equilibrium effects99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004., reaction limitations by the increase of mass transfer resistance, and so on. Moreover, it does not provide important information about the intrinsic kinetics of the process, which can help to elucidate the mechanism of the reaction88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
It is also important to realize that the colorimetric indicators, like phenolphthalein, are not able to provide a specific value of pH, since it has a characteristic range in which its color changes1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013.. Important errors can be added to the analysis, depending on the color of the analyzed medium and depending on the difficulty of the analyst in differentiating the exact titration equivalence point1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013.. Such errors are increased if the analyte is in low concentration, and this technique is particularly sensitive to the analyst experience in performing the procedure1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013..
In this sense, the objective of the present work is to analyze the kinetics of the pozzolanic reaction process, with three different silica nanoparticles reacting with calcium oxide in aqueous solutions. These materials present relative low-cost, what makes them economically attractive for the use as concrete additives. The studied samples were characterized and chemically tested, providing information about their morphology and about the kinetics of the reactions involved.
In order to help the elucidation of the reaction and to correct the temperature effects on the water ionization chemical equilibrium - the chemical equilibrium constant can reach values one order of magnitude higher than the value for 25 ºC (10-14) for temperatures lower than 100 ºC99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004. - the principles of the chemical equilibrium thermodynamics and the chemical kinetics are applied to the analysis. A particular mechanism is hypothesized, and it is shown that the test can be performed at lower temperatures and during less time than the ones used for Chapelle test, providing important energy savings and faster analysis. The use of an electronic, simple and non-expensive device - the pH meter - also provides more accuracy and confidence to the results obtained, consisting in a useful and fast methodology for the evaluation of the effectiveness of the pozzolanic addition in concrete.
Due to the wide use of concrete, silica, and other ceramic materials on the urban societies, it is of great importance to understand the intrinsic mechanisms that take place when they interact, making it possible to optimize the processes and to find alternatives friendlier in both the economic and environmental perspectives.
2. Materials and Methods
2.1 Thermodynamic analysis of the aqueous solution
In order to evaluate the dynamics of the pozzolanic reaction, the thermodynamic analysis of the aqueous medium must be analyzed properly and the corrections on pH scale with temperature must be performed. In the absence of such rigorous treatment, the simple reading of the pH meter can lead to results of hydroxide ions concentration one or two orders of magnitude lower than the actual values. For example, a reading of pH equals to 11.2 corresponds to a concentration of OH- ions of 0.0016 mol/L when the temperature is 25 ºC, but the value for the temperature of 75 ºC, used in this research, is approximately equals to 0.036 mol/L, more than 22 times higher.
For correcting the scales, the authors recommend a construction of a worksheet in a software like Microsoft Excel, with a column for the pH meter readings, a column for the experiment temperature, and a column for each one of the equations listed in the text. The equations constitute an algorithm that should be solved in the indicated order, and it is possible to build a computer routine in Excel VBA for solving the more complex operations, with the any adequate numerical method, such as Newton-Rhapson or even a try-and-error procedure and the equations variables are summarized in Table 1. The integral calculus can be performed using the trapezoidal rule, and all the parameters needed are given in the References. The authors recommend that the parameters are put in fixed cells, and the use of the sheet is not restricted to the pozzolanic analysis - it can be used for the temperature correction of the pH reading in any aqueous medium.
One of the reactants is the calcium oxide, which is known to elevate the water pH due to the liberation of hydroxide ions, accordingly to Equation 1 77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980.. The variation in OH- ions is then equals to the variation in CaO dissolved in water, and the CaO instant concentration can be calculated by interpreting the reading of a common electronic pH sensor, which gives the medium pH value when its probe is immersed in the aqueous suspension.
The water chemical equilibrium, expressed in Equation 2 77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980., is disturbed by the presence of the hydroxide ions formed, and the equilibrium equation relates the concentration of acid ions, detected by the pH meter, to the concentration of hydroxide ions.
The chemical equilibrium is described by the equilibrium constant, K, which correlates the chemical activities of the species in the medium, according to Equation 3 99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004..
In Equation 3, the indexes 1, 2 and 3 represent ion H(+), ion OH(-), and molecule H2O, respectively. The equilibrium constant is related to the reaction Gibbs energy by Equation 4, and the activity of each specie is related to its molar fraction in solution by Equation 5 99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004..
In a defined temperature T, it can be demonstrated from the classic van't Hoff equation that the variation in reaction enthalpy is given by Equation 6 99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004.. In this equation, ΔCp is the sum of each reactant and product specific heat multiplied by the respective stoichiometric coefficient, and T0 is a reference and arbitrary temperature.
The overall variation in reaction Gibbs energy, in a defined temperature, can be predicted from the variations in reaction enthalpy and in the reactants specific heat by Equation 7. This equation is derived from Equation 6, combined to the Kirchhoff and Gibbs equations99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004., where ΔG0 and ΔH 0 are the reaction Gibbs energy variation and enthalpy variation calculated in the reference temperature T0, respectively.
The values of ΔH/T are well known for the ionization equilibrium of water and are available for a wide range of temperatures1111 Olofsson G, Hepler LG. Thermodynamics of ionization of water over wide ranges of temperature and pressure. Journal of Solution Chemistry. 1974;4(2):127-143., allowing the calculation of the reaction ΔG with Equation 7. Then, the equilibrium constant can be calculated by applying this result to Equation 4.
As the water is always in large excess, with molar fractions above 0.9, its activity can be predicted by the Lewis-Randall rule1212 Lewis GN, Randall M. The thermodynamic treatment of concentrated solutions, and applications to thallium amalgams. Journal of the American Chemical Society. 1921;43(2):233-254., and it is equal to the water molar fraction:
The activity coefficient of ionic species can be evaluated using the Debye-Hückel model for excess Gibbs energy1313 Edwards TJ, Newman J, Prausnitz JM. Thermodynamics of aqueous solutions containing volatile weak electrolytes. AIChE Journal. 1975;21(2):248-259. , which is expressed by Equation 9.
The ionic strength, I, is defined by Equation 10. In the water system, the ionic charges are z1 = +1 and z2 = -1. Edwards et al.1313 Edwards TJ, Newman J, Prausnitz JM. Thermodynamics of aqueous solutions containing volatile weak electrolytes. AIChE Journal. 1975;21(2):248-259. recommend the use of the values β12 = β21 = 0.25 kg/mol for the interaction between ions hydrogen and hydroxide.
In order to evaluate the Debye-Hückel coefficient α, semi-empirical correlations can be applied, such as Equation 11 1414 Helgeson H, Kirkham D. Theoretical Prediction of the Thermodynamic Behavior of Aqueous Electrolytes at High Pressures and Temperatures: II. Debye-Hückel Parameters for Activity Coefficients and Relative Partial Molal Properties. American Journal of Science. 1974;274(10):1199-1261.. Water density and water dielectric constant are also known for a wide range of temperatures1515 Çengel YA, Cimbala JM. Fluid Mechanics: Fundamentals and Applications. McGraw-Hill; 2006. ,1616 Uematsu M, Frank EU. Static Dielectric Constant of Water and Steam. Journal of Physical and Chemical Reference Data. 1980;9(4):1291-1306..
After calculating the chemical activities in the reaction temperature, the species concentration can be evaluated by the application of the activity values in Equation 12, which is derived from Equations 3, 5 and 8.
It is important to note that, defining pH and pOH according to Equations 13 and 14 1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013., Equation 12 is simplified to the well-known Equation 15 1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013. when the reaction proceeds under 25 ºC.
2.2 Reaction experiments
In order to evaluate the kinetic behavior of a chemical reaction, it is necessary to analyze and to monitor a physical or chemical parameter which is related to the concentration of the species that are reacting88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. . Examples of such properties comprise the medium refractive index88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , pH88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , or viscosity1717 Bailey J, Ollis D. Biochemical Engineering Fundamentals. New York: McGraw-Hill; 1986..
In this research, reaction experiments were conducted in a bench-scale batch reactor made of glass, with magnetic stirring and external temperature control, and the mixture volume was fixed in 250 mL. The reactions were analyzed with a pH meter Gehaka PG 2000 able to measure pH and the sample temperature, equipped with electrode SC26 Sensoglass, adapted for handling temperatures up to 90 ºC (Gehaka, Brazil). The reactions were monitored until no more variation in pH could be observed. The medium was kept at constant temperature, but each experiment was carried out in a different condition, with temperatures varying from 55 to 80 ºC.
Three different sources of silica were used: amorphous silica nanoparticles ("soot"), microcrystalline quartz, and amorphous commercial "active silica" (Tecnosil, Brazil). These particles were characterized by scanning electron microscopy (SEM) and by X-Ray diffraction (XRD).
The pH values measured by the equipment were converted to hydrogen concentration by applying Equation 13, and the thermodynamic Equations 3 to 12 were applied for the conversion of these data in hydroxide ions concentration. The instant hydroxide concentration is related to the instant calcium oxide concentration by Equation 1, since CaO is the main responsible for the medium alkalinity.
2.3 Nanoparticles preparation
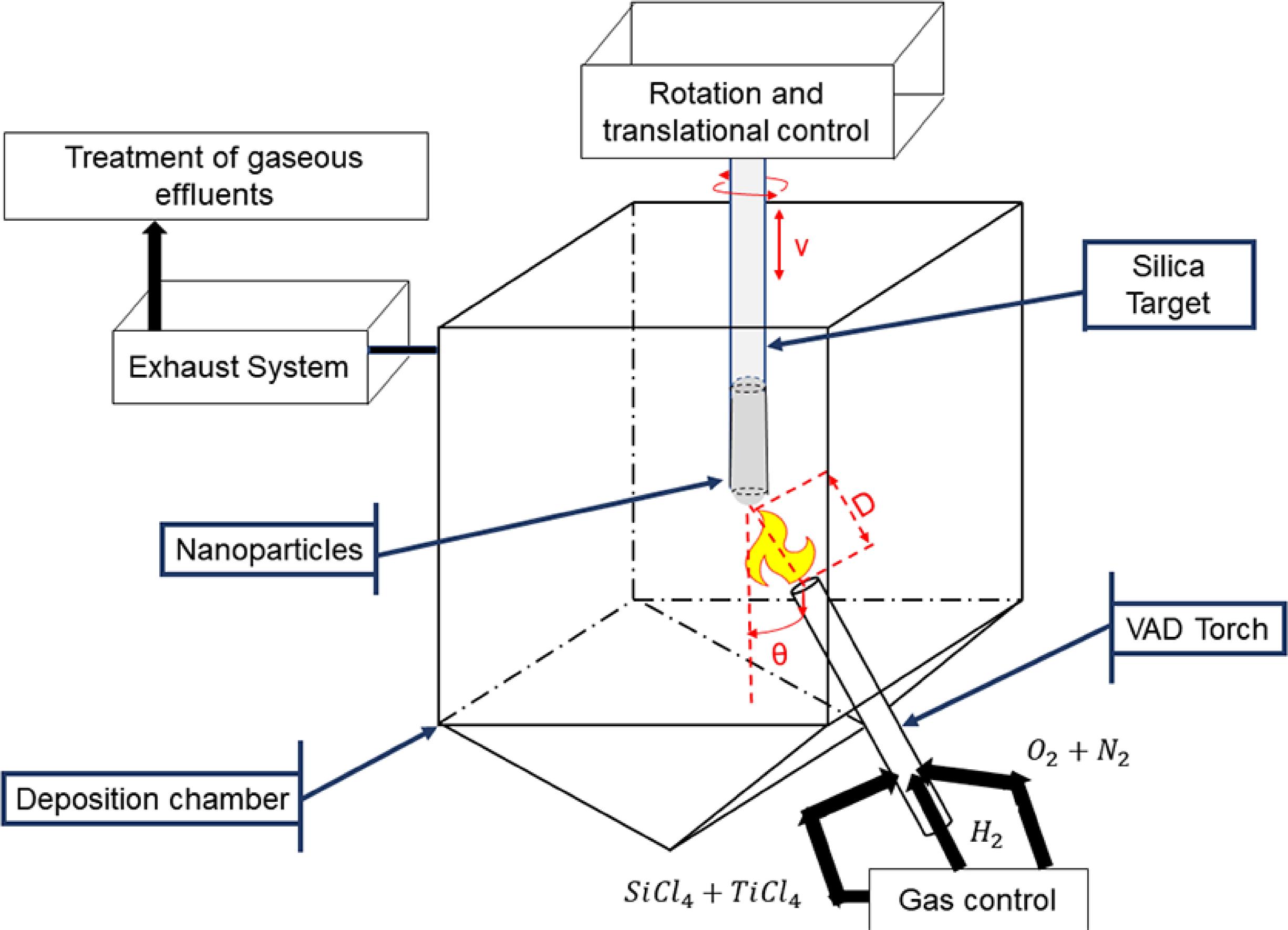
Soot particles were synthesized by the Vapor Axial Deposition (VAD) method, which has been widely studied for the production of optical fiber preforms1818 Santos JS, Ono E, Fujiwara E, Manfrim TP, Suzuki CK. Control of optical properties of silica glass synthesized by VAD method for photonic components. Optical Materials. 2011;33(12):1879-1883.. The silica particles produced by this methodology are completely amorphous and present spherical morphology1818 Santos JS, Ono E, Fujiwara E, Manfrim TP, Suzuki CK. Control of optical properties of silica glass synthesized by VAD method for photonic components. Optical Materials. 2011;33(12):1879-1883.. The particles morphologies can be precisely controlled by adjusting the gases flow rates, such as H2, O2, N2, and SiCl4 flow rates, and by controlling the burner-target distance D, the burner-target inclination angle θ, and the target vertical translation speed v1818 Santos JS, Ono E, Fujiwara E, Manfrim TP, Suzuki CK. Control of optical properties of silica glass synthesized by VAD method for photonic components. Optical Materials. 2011;33(12):1879-1883.,1919 Manfrim TP, Ono E, Fujiwara E, Santos MFM, Boery MNO, Santos JS, et al. A method to synthesize SiO2-TiO2 glasses based on the synergy between VAD and ALD techniques: study of TiO2 doping profile along radial direction. Optical Materials. 2011;33(12):1938-1942., as shown in Figure 1.
A high-temperature O2-H2 flame promotes the hydrolysis and oxidation of SiCl4, according to reaction 16. The nanoparticles are axially deposited on the surface of a silica rod target in constant rotation and controlled vertical translation movements1919 Manfrim TP, Ono E, Fujiwara E, Santos MFM, Boery MNO, Santos JS, et al. A method to synthesize SiO2-TiO2 glasses based on the synergy between VAD and ALD techniques: study of TiO2 doping profile along radial direction. Optical Materials. 2011;33(12):1938-1942..
After the deposition, a "green body" is formed over the silica rod, as shown in Figure 2. This green body is mechanically separated from the rod and crushed in a silica mortar in order to separate the agglomerated nanoparticles, forming the silica powder (soot).
The crystalline quartz microparticles were produced in-house using ball milling in silica medium, and quartz lumps were used as balls, in order to minimize the material contamination2020 Fecht HJ, Hellstern E, Fu Z, Johnson WL. Nanocrystalline metals prepared by high-energy ball milling. Metallurgical Transactions A. 1990;21:2333-2337.. The mill is loaded with the quartz sand and deionized water and is subjected to constant rotation (fill factor K= 0.42)2121 Sopicka-Lizer M, ed. High-Energy Ball Milling: Mechanochemical Processing of Nanopowders. Cambridge: Woodhead Publishing; 2010. . The quartz balls comminute the sand and progressively reduce the crystal dimensions. The milling time was fixed in 165 h, and the rotation speed was set to 100 rpm2222 Fujiwara E, Schenkel EA, Santos MFM, Suzuki CK. Concentration measurements in silica and quartz nanofluids by optical fiber sensor. In: SPIE Proceedings 9634; 24th International Conference on Optical Fibre Sensors; 2015 Sep 28-Oct 2; Curitiba, PR, Brazil..
After the milling process, the mixture of milled quartz and water was transferred to a 2 L graduated cylinder and completed with deionized water. After 48 h, the large particles were sedimented, whereas submicron particles remained floating in the translucid phase, as shown in Figure 3. Then, this clarified portion was separated and the water was evaporated, forming a fine powder, yielding micro and nanostructured crystals.
Decanting process. After 48h, the translucent phase is drained and the water is evaporated, forming a powder containing submicron particles.
2.4 Mechanistic hypothesis and integral analysis
Since in standard temperature and pressure conditions (STP) silica can be classified as an acid oxide, weakly ionizable in water, getting a negative charge77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980., and calcium oxide is a basic oxide, as shown in Equation 1, it can be hypothesized that the pozzolanic reaction consists in a Lux-Flood acid-base reaction2323 Lee JD. Concise Inorganic Chemistry. Hoboken: Wiley-Blackwell; 1999..
Lux-Flood concept defines acid as a species which is able to receive oxide ions, whereas a base is a species which is able to donate oxide ions2323 Lee JD. Concise Inorganic Chemistry. Hoboken: Wiley-Blackwell; 1999.. Lux-Flood definition is mainly applied to fused oxide and fused salt systems and can be understood as the neutralization of an acid oxide by a basic oxide77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980.,2323 Lee JD. Concise Inorganic Chemistry. Hoboken: Wiley-Blackwell; 1999..
It is important to realize that, in aqueous solutions, the Lewis acid-base definition, by which the base is a species able to donate an electron pair and an acid is a specie able to receive it77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980.,2323 Lee JD. Concise Inorganic Chemistry. Hoboken: Wiley-Blackwell; 1999. can be equivalently applied to this system. Both approaches lead to Equation 17 as the representation of the process, in a single-step reaction. The presence of crystallization water was not considered, since the salt hydration degree varies with the temperature and with the crystallization conditions77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980.,2323 Lee JD. Concise Inorganic Chemistry. Hoboken: Wiley-Blackwell; 1999..
According to Equation 17, the calcium oxide concentration reduces during the reaction, so it is expected that the medium pH reduces while the reaction proceeds, and then remains approximately constant when the limiting reactant is completely consumed.
In order to evaluate the consistency of this hypothesis, it is important to perform the integral analysis of the kinetic data. As extensively described by Levenspiel88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , the integral method consists of integrating the kinetic expression proposed for a particular mechanism, and then comparing this theoretical curve of concentration varying with time with the experimental values.
If the proposed mechanism is correct, then there must be a satisfactory correspondence between the integral curve and the data obtained. Since the integral analysis is based on theoretical principles, the result provides strong evidence for supporting a particular reaction mechanism88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
Defining the chemical species A as CaO, species B as SiO2, and the ratio M as the relation between the initial concentration of CaO to the initial concentration of SiO2, it can be demonstrated that, if Equation 17 is correct, then the instant concentration of CaO is described by Equation 18 88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
Equation 18 applicability is limited to the situations where M ≠ 1 and gives a linear plot when its left side, ln[(M-xA)/M(1-xA)], is graphically correlated with the reaction time. If the experimental data is satisfactorily fitted by the straight line described by Equation 18, the fitting angular coefficient is equals to CA0(M-1)k. The adjusted curve usually shows a residual linear coefficient which is not related to the particular chemical kinetics but is due to random experimental errors88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
When using the methodology for the special case where M = 1, the expression which must be valid if Equation 17 is correct is the Equation 19. This expression gives a linear plot when its left side is graphically correlated with reaction time. Now, the angular coefficient is equals to k, and the linear coefficient is equals to 1/CA088 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
Since there is significant difference depending on the value of M, experiments should be performed under conditions in which M = 1, or in which M is significantly different from 188 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. . Then, the experiments in this study were performed under conditions in which M ≈ 0.25.
The experiments are summarized in Table 2. Calcium oxide was chosen as the limiting reactant, since this would provide the highest variation in pH, enhancing the sensor sensitivity and allowing the correct detection of the reaction dynamics. Silica masses values were chosen in accordance with the following criteria: (1) the silica masses should be in large excess, but the value of M should be maintained constant, for providing a simpler integral analysis; (2) the masses or proportions of reactants should be in accordance to the values used in studies applying Chapelle test; (3) since one of the objectives of this research was to provide savings of energy and use of chemical reactants, the values should be as lower as possible, but sufficiently large for maintaining the sensor capability in detecting pH variations during the reactions.
3 Results and Discussion
3.1 Materials characterization
The three silica materials were analyzed by SEM technique in Brazilian Nanotechnology National Laboratory (LNNano) microscope (FEI Quanta 650 FEG, USA), and the particles dimensions were graphically evaluated using the Image Viewer application of MATLAB 2016 (Mathworks, USA), an image processing routine that is capable of performing the quantification and measurement of different objects and shapes in a particular image. The SEM samples were prepared by depositing the powder over a conductive carbon double-sided tape fixed in the stub. The samples were also carbon coated using a Bal-Tec SCD 050 Sputter Coater to obtain images under a high vacuum regime.
The XRD analyses were performed using a Rigaku DMAX 2200 diffractometer (Rigaku, Japan), with fine focus Cu tube (λ = 1.54 Å), Bragg-Bretano geometry (θ-2θ), 0.02º step and 2.5 s/step exposition time.
The quartz particles, shown in Figure 4, presented non-spherical morphologies, with considerable dispersion in their average dimensions (Table 3), which was expected due to the comminution process2121 Sopicka-Lizer M, ed. High-Energy Ball Milling: Mechanochemical Processing of Nanopowders. Cambridge: Woodhead Publishing; 2010. ,2424 Chen M, McCauley JW, Hemker KJ. Shock-induced localized amorphization in boron carbide. Science. 2003;299(5612):1563-1566. ,2525 Koch CC. Top-Down Synthesis of Nanostructured Materials: Mechanical and Thermal Processing Methods. Reviews on Advanced Materials Science. 2003;5(2):91-99..
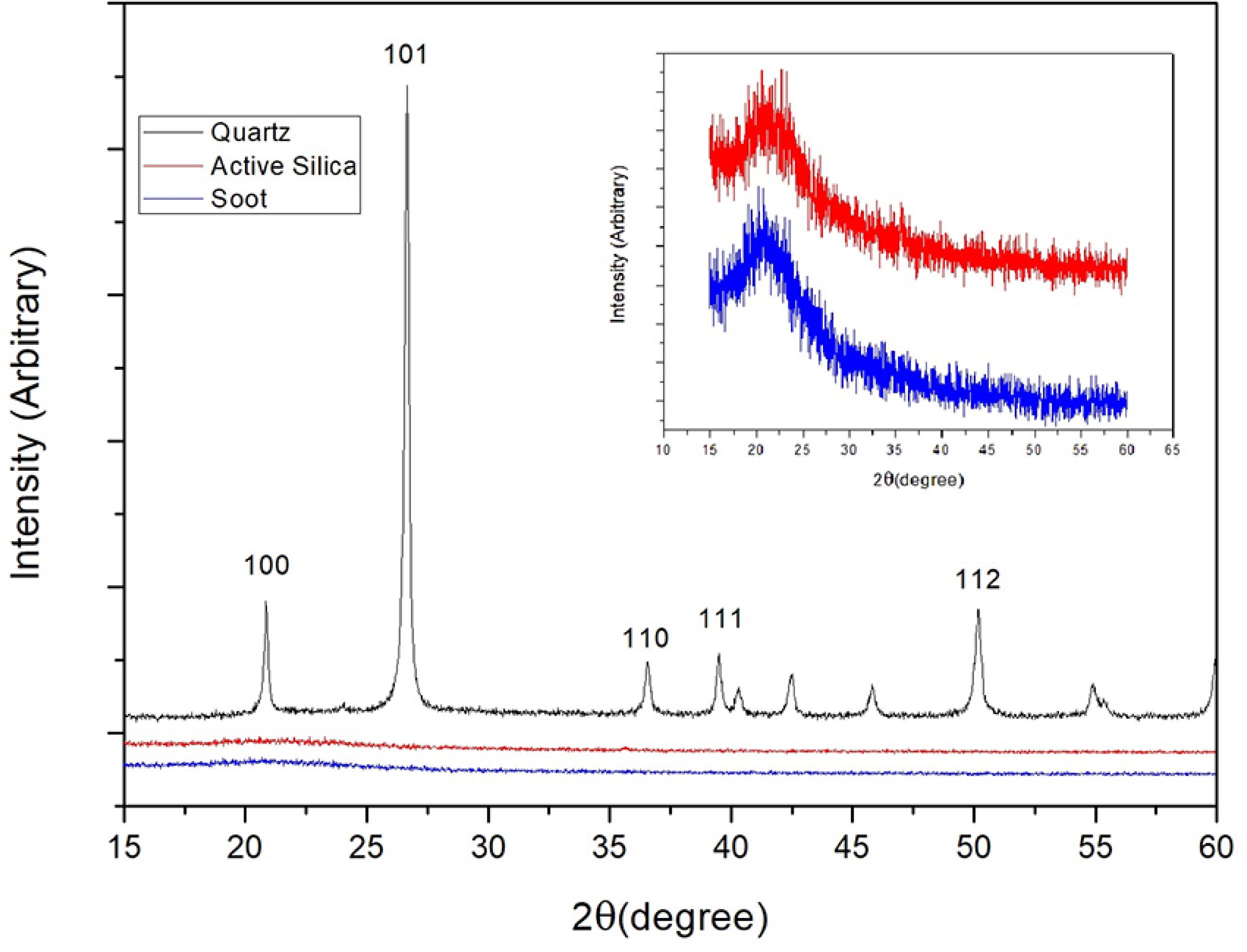
According to the quartz XRD pattern, shown in Figure 5, the sample is comprised of practically α-quartz, with the absence of amorphous halo. This same figure also shows that active silica and soot are essentially amorphous, with a low-intensity crystalline peak near 2θ = 20 º.
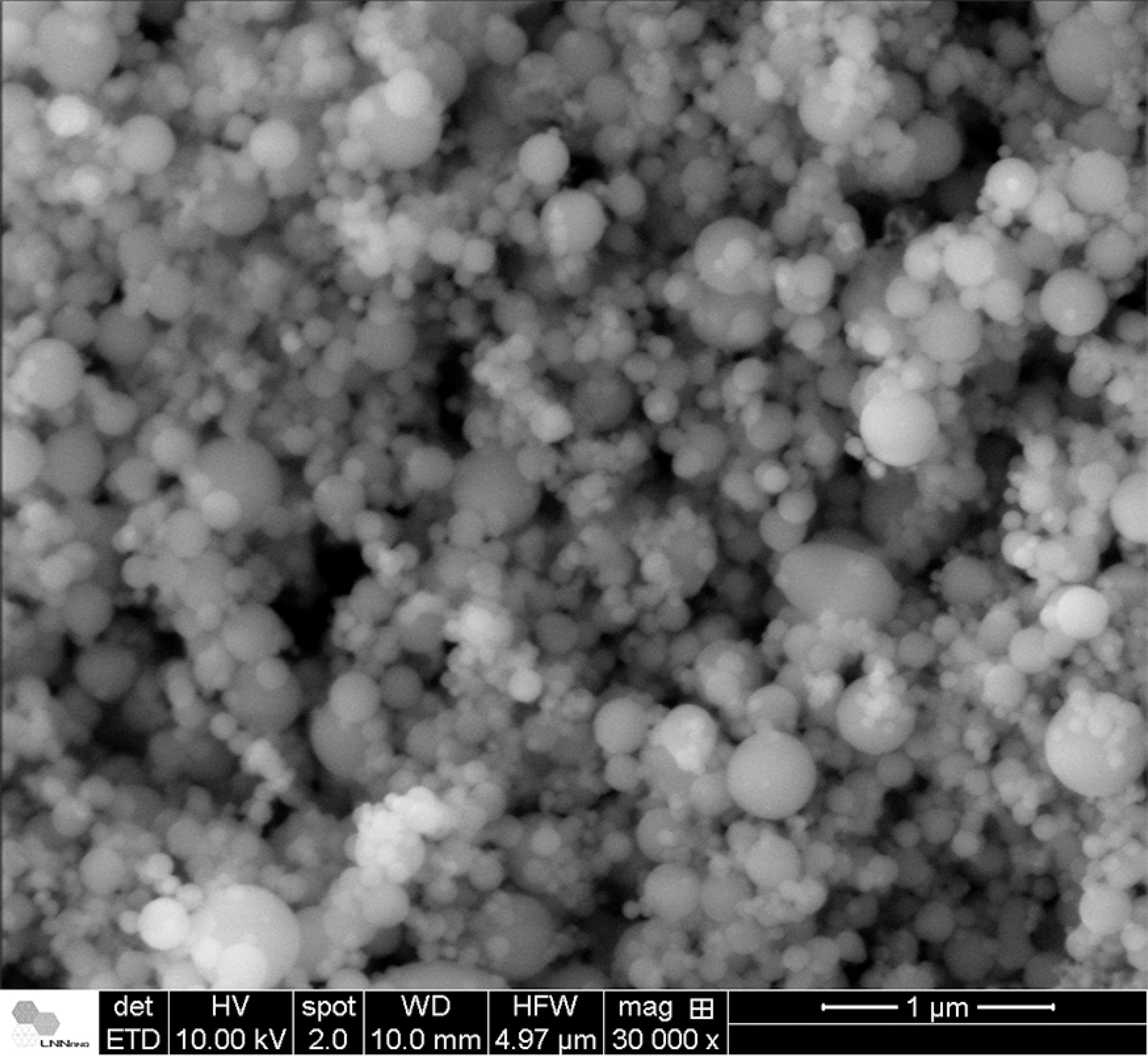
The SEM images obtained for the soot and for the active silica are shown in Figure 6 and Figure 7, respectively, and indicate spherical particles with diameters in the range of nanometers (Table 3). Active silica particles also showed a broader diameter distribution in comparison to the soot, what is expected from the fact that the VAD process is capable of providing a finer control regarding the morphology of produced nanoparticles1818 Santos JS, Ono E, Fujiwara E, Manfrim TP, Suzuki CK. Control of optical properties of silica glass synthesized by VAD method for photonic components. Optical Materials. 2011;33(12):1879-1883..
XRD pattern obtained for submicron quartz. The inset shows the soot and the active silica low-intensity crystalline peaks.
3.2 Kinetic experiments
The experimental results are summarized in Table 4, which presents the reaction time, initial pH, the total pH variation observed, the total conversion of calcium oxide and the linear correlation coefficient, R2, obtained for the application of Equation 18.
It is important to note that, after the mixture of the reactants, the system can experience a "dead-time", when no reaction is noticeable. This time must not be considered for the linear plot analyses, since the integral method is based on the assumption that the chemical reaction starts as soon as the reagents are put in contact88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
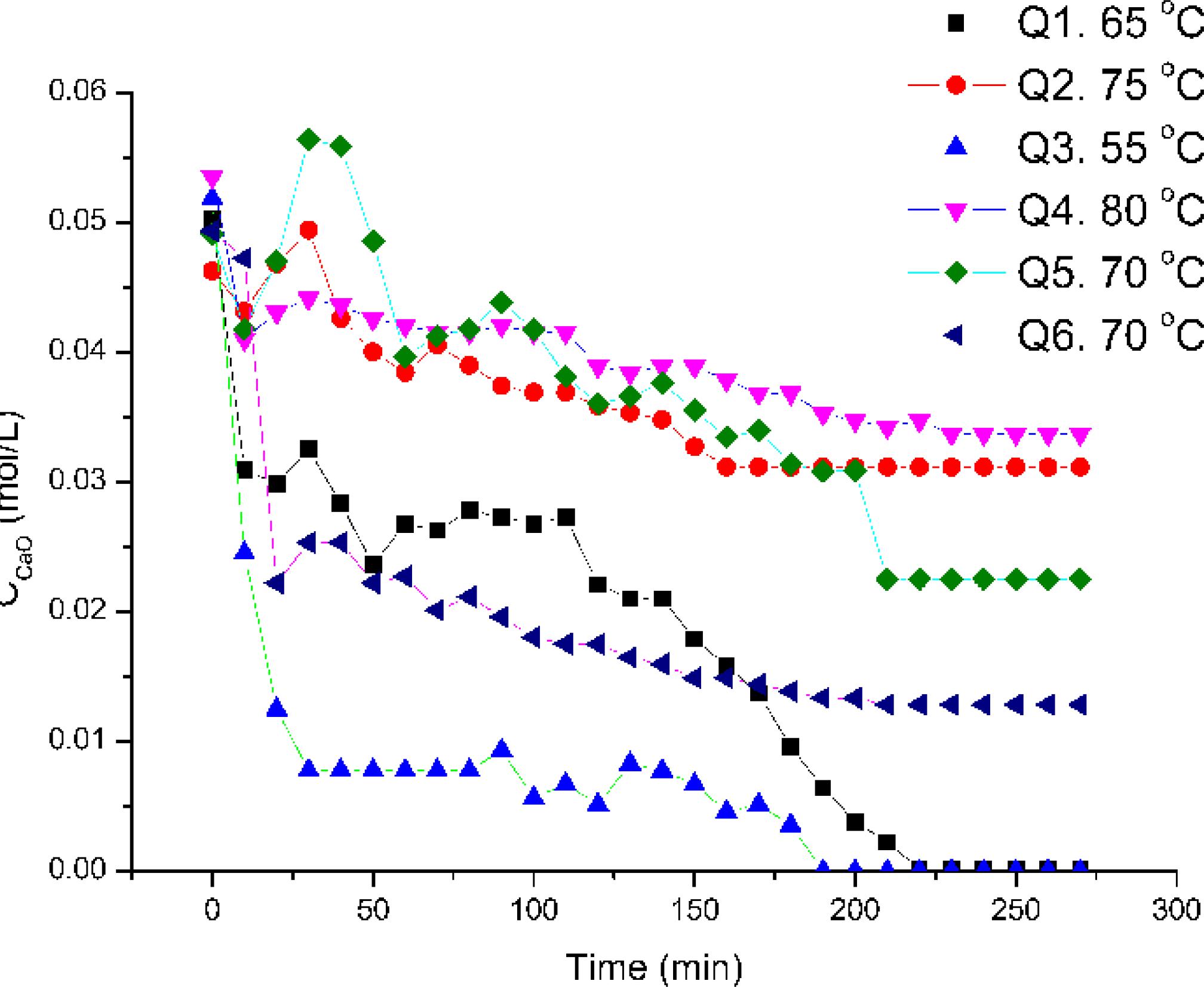
From Figures 8 to 10, it is possible to notice that the concentration of calcium oxide reaches a constant value in a relatively short time interval. In most of the reactions, the chemical process is not able to convert all of the CaO, and the medium is still alkaline after the end of the reaction. It proves that not all of the silica is in acidic form, being not able to react with the base dissolved in liquid medium.
Kinetic experiments conducted with crystalline quartz as the silica source. The lines are guides for the eye.
Kinetic experiments conducted with soot as the silica source. The lines are guides for the eye.
Kinetic experiments conducted with active silica as the silica source. The lines are guides for the eye.
The graphics show a classical pattern obtained when a chemical equilibrium is reached99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004.. This fact is in accordance with the silica chemical properties, since the acidic characteristics of the silica are related to its superficial free silanol concentration2626 Iler RK. The Chemistry of Silica Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. Hoboken: John Wiley and Sons; 1978.,2727 Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;173(1-3):1-38., and the formation and consequently reactivity of this group is represented by a chemical equilibrium between silica and water2626 Iler RK. The Chemistry of Silica Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. Hoboken: John Wiley and Sons; 1978.,2727 Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;173(1-3):1-38.. The consequence is that, depending on the particular conditions of the medium, not all of the silanols are able to react as acid groups, and such acid groups are in equilibrium with neutral groups2626 Iler RK. The Chemistry of Silica Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. Hoboken: John Wiley and Sons; 1978.,2727 Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;173(1-3):1-38..
It is important to realize that, when the reaction is complete, the system pH is considerably higher than 8, which is the approximate turning point of the phenolphthalein indicator at 25 ºC1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013.. It can be expected errors in the results for titrations if they are conducted at the same temperature of the reaction systems, higher than 25 ºC, and it is also important to analyze if the equilibrium between the two different ionization states of the indicator1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013. is substantially affected by the temperature.
This variation would change the turning point, and a complete thermodynamic analysis would be necessary to verify if the equivalence point coincides with the indicator turning point1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013.. Even if the titration temperature is rigidly controlled under 25 ºC, the reversibility of the calcium oxide reaction can disturb the analysis since, according to Equations 3, 4 and 7, the species concentration in equilibrium can change with the variation of the temperature88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. ,99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004..
The solutions for Equations 3, 4 and 7 also show that, for temperatures higher than 55 ºC, the neutral concentration of hydroxide ions is at least one order of magnitude higher than for 25 ºC. The conclusion is that the neutral pH, where hydrogen and hydroxide ions concentrations are equal, is considerably higher than 7. As the Chapelle test does not consider this factor44 Perraki T, Kakali G, Kontoleon F. The effect of natural zeolites on the early hydration of Portland cement. Microporous and Mesoporous Materials. 2003;61(1-3):205-212.,66 Kakalia G, Perraki T, Tsivilis S, Badogiannis E. Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Applied Clay Science. 2001;20(1-2):73-80., one can expect several deviations between the "reactivity" calculated by this test and the real value of the reaction yield.
An accurate analysis of the results of the Chapelle test is difficult, since the medium temperature is increased until the aqueous solution reaches its bubble point44 Perraki T, Kakali G, Kontoleon F. The effect of natural zeolites on the early hydration of Portland cement. Microporous and Mesoporous Materials. 2003;61(1-3):205-212.,66 Kakalia G, Perraki T, Tsivilis S, Badogiannis E. Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Applied Clay Science. 2001;20(1-2):73-80.. As the bubble point is a strong function of the local atmospheric pressure, and of the particular chemical species in the medium and their concentrations99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004., there is no standardization in the test temperature and, depending on all of these conditions, very specific reaction rates and equilibrium states will be obtained for the reaction88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. ,99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004.. Since such factors change the observed yield, it will not be possible to compare results from different Chapelle test experiments, even when real experimental temperatures are documented.
The reaction times given in Table 4 are the time differences between the first instant when the concentration shows variation and the instant when the final plateau is reached, evidencing the end of the reaction. For each one of the three different silica sources, the mean values of the reaction times were evaluated with the ANOVA ("analysis of variance"). It is a statistical procedure for evaluating the confidence intervals of the mean results obtained for different samples and the significance of the differences between different means. If the confidence intervals show no intersections, it is possible to conclude with a given confidence - in this research, 95% confidence - that the mean results are statistically different.
Figure 11 shows the 95% confidence intervals of the mean reaction times observed for the crystalline quartz tests, for the soot tests, and for the active silica tests, obtained with the ANOVA test performed with 95% confidence, and Table 5 summarizes the values of the mean reaction times and the calculated confidence intervals. The analysis was performed in Minitab 17 (Minitab Inc, USA) and indicates a significant difference in the mean reaction time observed for crystalline quartz, which is considerably higher than the observed for the other silica sources, and no significant difference between the mean reaction times observed for active silica and for soot tests.
ANOVA test of the reaction times, with 95% confidence interval (CI) for mean reaction time. The lines connecting dots and the horizontal lines are guides for the eye.
In all of the experiments shown in Figure 8, there is an initial baseline before calcium oxide concentration varies significantly, and it is a determinant factor for the higher reaction time. As the silica amorphous materials have a lower reaction time, a feasible hypothesis is that the crystalline structure is responsible for the lower reaction rate.
It is possible that, before it is able to react with the free basic species dissolved in the liquid medium, the silica silanol groups must be exposed in the surface, and the rigidity of the crystalline structure reduces the number of available groups2626 Iler RK. The Chemistry of Silica Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. Hoboken: John Wiley and Sons; 1978.,2727 Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;173(1-3):1-38.. Then, the initial baseline would be caused by the combined effect of the temperature, the electric interaction between the silica surface and the hydroxide negatively charged ions, and the liquid fluid dynamics.
These effects would be responsible for inducing the presence of silanol groups in silica surface, in accordance with the Zhuravlev model for silica chemistry2727 Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;173(1-3):1-38.. Then, the effective reaction only starts when there are sufficient acid groups able to neutralize the base.
Another important possibility is the effect of the surface area - it is a general principle in chemical kinetics that the reaction rate rises with the increase in surface area88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
Figures 4, 6 and 7 and the data presented in Table 3 evidence that the crystalline quartz shows a higher value of particle mean diameter, a lower value of sphericity and also has a lower specific surface area, what decreases the interaction area between the silica particles and the CaO, reducing the rate of reaction, according to the Langmuir theory88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. . The quantification of the adsorption sites on silica surface and the specific area of the particles needs a specific BET study88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , and the analysis of this effect is an important limitation of the presented methodology. With the proposed instrumentation, it can only be performed qualitatively when there are available SEM images which evidence the differences in the specific area.
In most of the experiments conducted with soot and active silica, the reaction reaches the equilibrium plateau in a very short time, making it difficult to compare the results for different temperatures, since the temperature effect has little significance in these cases.
However, in quartz experiments, in which the time scale is wider, the tendency observed is in accordance with the hypothesis made. The initial reaction rate is increased with temperature, and both the Equation 17 and the analysis of Lux-Flood and Lewis theories suggest an acid-base neutralization reaction.
It is commonly observed that acid-base reactions are considerable exothermic77 Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. Hoboken: John Wiley and Sons; 1980.
8 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999.
9 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004.-1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013., and the chemical equilibrium in reactions with such thermodynamic behavior is favored by lower temperatures88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. ,99 Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics. New York: McGraw-Hill; 2004..
Figure 8 shows the correspondence between the total CaO converted and this hypothesized temperature behavior: the two lowest temperatures, 65 and 55 ºC, show complete conversion; the two experiments conducted at 70 ºC show a small variation, expected due to stochastic effects, and both cases show higher conversions than the other two experiments conducted in higher temperatures. The experiment performed at 75 ºC exhibits a lower conversion than the experiments conducted at 70 ºC, but a higher conversion than the experiment carried out at 80 ºC. Finally, the experiment conducted at 80 ºC, the highest temperature tested, shows the lowest total conversion of CaO.
Table 6 summarizes a comparison between the analyses performed with the methodology proposed in this research and the Chapelle test. It can be easily concluded that there are advantages related to time, energy consumed, and to more accuracy and confidence obtained in the experiments.
3.3 Mechanistic integral analysis
The fitting of the experimental values to the straight line described by Equation 18 was applied in order to verify if the kinetic data obtained was consistent with the proposed chemical mechanism. The linear correlation coefficient obtained for the adjusted straight lines were R2 ≅ 1 for all of the experiments, and this fact reveals a strong linear correlation and consistency with the hypothesis. It is worth noticing that the coefficient could not be calculated for experiment AS4 due to the low reaction time, since it is necessary to remove both the dead-time and the plateau, as it was already mentioned.
Figure 12 shows the straight lines F(t), defined by Equation 18, obtained for 4 experiments using crystalline quartz, and Figure 13 shows the straight lines obtained for 1 experiment using soot and for 2 experiments using active silica as the silica source.
Straight lines obtained for four of the experiments using crystalline quartz, in the integral analysis.
Straight lines obtained for one experiment using soot and for two experiments using active silica, in the integral analysis.
The data show a complete agreement with the expected behavior, and it is possible to observe that some experiments for active silica obtained R2 > 0.98, what is technically a perfect straight line. The deviations from the linear regression can be attributed to stochastic errors during the experiments.
The fitted straight lines F(t) obtained can be described by their angular and linear coefficients, and the angular coefficient is related to the kinetic rate constant k in each experimental temperature accordingly to Equation 18. Table 7 summarizes the coefficients obtained for each one of the linear adjustments, even the ones that were not shown in Figures 12 and 13, but the analysis could not be performed for experiment AS4 due to the low number of data collected. The main result showed in Table 7 is the consistency of the data, that show similar values and order of magnitudes for the same parameters. This data is important for reinforcing the kinetic hypothesis, a 1:1 reaction.
Angular and linear coefficients of Equation 18 and numerical values of the rate constants obtained for each experiment.
It is not possible to observe neither clear differences in the mean values of the parameters nor a tendency of the kinetic constants with the increase in the temperature range tested but, as it was already cited, a complete study of the correlation between kinetics and the specific surface area should be performed in future works in order for determining possible correlations.
4 Conclusions
The integral analysis provided semi-empirical evidence for the proposed mechanism, a 1:1 reaction, since it is very unusual for a chemical reaction to agree with more than one integral straight line88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , and the satisfactory adjustment to the proposed model was obtained for 16 experiments performed under different conditions. It is important, however, to emphasize that, due to the very small scales of dimensions and time events, it is very difficult to prove that a particular mechanism is valid for generalized cases, since the events take place in the molecular and atomic levels88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. . A proposed mechanism can be accepted as a right and consistent one until unquestionable facts against it have been discovered88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. .
The statistically significant difference of mean reaction times obtained for different silica sources provided evidence that the crystalline structure and the particles specific surface area can be determinant factors for the reaction rates, and these hypotheses are supported by the silica model developed by Zhuravlev2727 Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;173(1-3):1-38. for the chemistry of silanol groups in water, and by the Langmuir kinetic model88 Levenspiel O. Chemical Reaction Engineering. Hoboken: John Wiley and Sons; 1999. , respectively. It was verified a reaction time of 195 min for crystalline silica particles with average diameter of 0.66 µm, and reaction time of 61 min for active silica particles with average diameter of 243 nm. Both reaction times are very lower than the standard time of the Chapelle test, 16 hours plus the time required for the titration chemical analysis.
It is important to realize that the proposed analysis has many advantages in comparison to the traditional Chapelle test. Besides the less time and energy-consuming, the procedure prevents the analysis to be affected by parallel reactions, such as the reaction with carbon dioxide from atmospheric air, and takes in account the effects of the temperature, allowing the comparison between experiments performed in very different conditions, since the reaction parameters are corrected by rigorous thermodynamic equations. It is also able to eliminate the imprecisions related to the visual titration technique, that can show unsatisfactory results in excessive turbid media, and the fluctuations related to the poor temperature control. It is possible to evaluate the temperature effects on the reversibility of the reaction as well, but there is an intrinsic limitation in evaluating the effect of the particles surface area, which needs a separate BET study.
The next studies should then evaluate the specific surface area effect on chemical reaction rate and evaluate if temperatures even lower than 55 ºC can be employed for the analysis, depending on the granulometry, providing even more energy savings and helping the elucidation of the effects of the surface energy on interactions between nanoparticles.
The titration with chemical indicators which change their colors in the turning point - used in Chapelle test - is a classic analytical procedure, but it is particularly sensitive to the analyst experience and familiarity with the technique1010 Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Pacific Grove: Brooks Cole; 2013.. In this sense, the use of an electronic, simple and non-expensive device, such as the pH meter, provides more accuracy and confidence to the results obtained and important time savings, being more robust in relation to the possible errors of the experimenters.
5. Acknowledgements
The authors would like to thank Brazilian Nanotechnology National Laboratory LNNano) for the use of the facilities, CNPq, CAPES, and FAPESP Grants 2015/02185-3 and 2017/20445-8 for financial support.
6. References
-
1Poon CS, Lam L, Kou SC, Wong YL, Wong R. Rate of pozzolanic reaction of metakaolin in high-performance cement pastes. Cement and Concrete Research 2001;31(9):1301-1306.
-
2Caldarone MA, Gruber KA, Burg RG. High Reactivity Metakaolin (HRM): A New Generation Mineral Admixture for High Performance Concrete. Concrete International 1994;16(11):37-40.
-
3Zhang MH, Malhotra VM. Characteristics of a thermally activated alumino-silicate pozzolanic material and its use in concrete. Cement and Concrete Research 1995;25(8):1713-1725.
-
4Perraki T, Kakali G, Kontoleon F. The effect of natural zeolites on the early hydration of Portland cement. Microporous and Mesoporous Materials 2003;61(1-3):205-212.
-
5Sabir BB, Wild S, Bai J. Metakaolin and calcined clays as pozzolans for concrete: a review. Cement and Concrete Composites 2001;23(6):441-454.
-
6Kakalia G, Perraki T, Tsivilis S, Badogiannis E. Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Applied Clay Science 2001;20(1-2):73-80.
-
7Cotton FA, Wilkinson G. Advanced Inorganic Chemistry Hoboken: John Wiley and Sons; 1980.
-
8Levenspiel O. Chemical Reaction Engineering Hoboken: John Wiley and Sons; 1999.
-
9Smith JM, Van Ness HC, Abbott MM. Introduction to Chemical Engineering Thermodynamics New York: McGraw-Hill; 2004.
-
10Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry Pacific Grove: Brooks Cole; 2013.
-
11Olofsson G, Hepler LG. Thermodynamics of ionization of water over wide ranges of temperature and pressure. Journal of Solution Chemistry 1974;4(2):127-143.
-
12Lewis GN, Randall M. The thermodynamic treatment of concentrated solutions, and applications to thallium amalgams. Journal of the American Chemical Society 1921;43(2):233-254.
-
13Edwards TJ, Newman J, Prausnitz JM. Thermodynamics of aqueous solutions containing volatile weak electrolytes. AIChE Journal 1975;21(2):248-259.
-
14Helgeson H, Kirkham D. Theoretical Prediction of the Thermodynamic Behavior of Aqueous Electrolytes at High Pressures and Temperatures: II. Debye-Hückel Parameters for Activity Coefficients and Relative Partial Molal Properties. American Journal of Science 1974;274(10):1199-1261.
-
15Çengel YA, Cimbala JM. Fluid Mechanics: Fundamentals and Applications McGraw-Hill; 2006.
-
16Uematsu M, Frank EU. Static Dielectric Constant of Water and Steam. Journal of Physical and Chemical Reference Data 1980;9(4):1291-1306.
-
17Bailey J, Ollis D. Biochemical Engineering Fundamentals New York: McGraw-Hill; 1986.
-
18Santos JS, Ono E, Fujiwara E, Manfrim TP, Suzuki CK. Control of optical properties of silica glass synthesized by VAD method for photonic components. Optical Materials 2011;33(12):1879-1883.
-
19Manfrim TP, Ono E, Fujiwara E, Santos MFM, Boery MNO, Santos JS, et al. A method to synthesize SiO2-TiO2 glasses based on the synergy between VAD and ALD techniques: study of TiO2 doping profile along radial direction. Optical Materials 2011;33(12):1938-1942.
-
20Fecht HJ, Hellstern E, Fu Z, Johnson WL. Nanocrystalline metals prepared by high-energy ball milling. Metallurgical Transactions A 1990;21:2333-2337.
-
21Sopicka-Lizer M, ed. High-Energy Ball Milling: Mechanochemical Processing of Nanopowders Cambridge: Woodhead Publishing; 2010.
-
22Fujiwara E, Schenkel EA, Santos MFM, Suzuki CK. Concentration measurements in silica and quartz nanofluids by optical fiber sensor. In: SPIE Proceedings 9634; 24th International Conference on Optical Fibre Sensors; 2015 Sep 28-Oct 2; Curitiba, PR, Brazil.
-
23Lee JD. Concise Inorganic Chemistry Hoboken: Wiley-Blackwell; 1999.
-
24Chen M, McCauley JW, Hemker KJ. Shock-induced localized amorphization in boron carbide. Science 2003;299(5612):1563-1566.
-
25Koch CC. Top-Down Synthesis of Nanostructured Materials: Mechanical and Thermal Processing Methods. Reviews on Advanced Materials Science 2003;5(2):91-99.
-
26Iler RK. The Chemistry of Silica Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry Hoboken: John Wiley and Sons; 1978.
-
27Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2000;173(1-3):1-38.
Publication Dates
-
Publication in this collection
25 June 2018 -
Date of issue
2018
History
-
Received
20 Feb 2018 -
Reviewed
06 Apr 2018 -
Accepted
11 May 2018