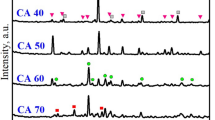
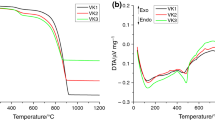
Abstract
Calcium silicate based cementitious materials are well known by its reactivity with carbon dioxide to produce high early strength through carbonation curing. Since the early strength gain is due to the reaction between calcium silicates and carbon dioxide, carbonation cement can be formulated with calcium silicates of any polymorph with reduced synthesizing temperature. Its early strength is expected from carbonation activation and late strength by hydration reaction. Such a cement can be made exclusively with industry wastes at low temperature. This paper is to examine the feasibility of synthesizing carbonation cement for carbonation process from two industry wastes: ladle slag fines for calcium and waste glass for silica. A synthesis process involving burning the mixture of ladle slag and waste glass was developed to produce low energy carbonation cement capable of gaining strength when activated by carbonation. The glass to slag ratio and clinkering temperature were determined based on carbonation and hydration strength of the binder. Performance was assessed by compressive strength tests, X-ray fluorescence analysis, X-ray diffraction analysis, and thermogravimetry analysis. The results suggested that supplementing the ladle slag with additions of waste glass had promoted the conversion of calcium to more value-added calcium silicate phases which could be activated by carbon dioxide to produce early strength. The carbonation cement so produced consumes no natural resources and has shown the potential for carbon sequestration.








Similar content being viewed by others
Abbreviations
- CaO:
-
Calcium oxide
- SiO2 :
-
Silica oxide
- H2O:
-
Water
- CO2 :
-
Carbon dioxide
- CaCO3 :
-
Calcium carbonate
- C2S:
-
Dicalcium silicate
- C3S:
-
Tricalcium silicate
References
Worrell E, Price L, Martin N, Hendriks C, Meida LO (2001) Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ 26:303–329
Kim Y, Worrell E (2002) CO2 emission trends in the cement industry: an international comparison. Mitig Adapt Strateg Glob Chang 7:115–133
Ishak SA, Hashim H (2015) Low carbon measures for cement plant—a review. J Clean Prod 103:260–274
Georgescu M, Tipan J, Badanoiu L, Crisan D, Dragan I (2000) Highly reactive dicalcium silicate synthesised by hydrothermal processing. Cem Concr Compos 22:315–319
Guerrero A, Goni S, Macias A, Luxan MP (1999) Mechanical properties, pore size distribution and pore solution of fly ash belite cement mortar. Cem Concr Res 29:1753–1758
Jiang W, Roy DM (1992) Hydrothermal processing of new fly ash cement. Ceram Bull Am Ceram Soc 71:642–647
Kacimi L, Simon-Masseron A, Salem S, Ghomari A, Derriche Z (2009) Synthesis of belite cement clinker of high hydraulic reactivity. Cem Concr Res 39:559–565
Popescu CD, Muntean M, Sharp JH (2003) Industrial trial production of low energy belite cement. Cem Concr Compos 25:689–693
Singh N (2006) Hydrothermal synthesis of b-dicalcium silicate (b-Ca2SiO4). Prog Cryst Growth Charact 52:77–83
Raupp-Pereira F, Ball RJ, Rocha J, Labrincha JA, Allen GC (2008) New waste based clinkers; belite and lime formulations. Cem Concr Res 38:511–521
Young J, Berger R, Breese J (1974) Accelerated curing of compacted calcium silicate mortars on exposure to CO2. J Am Ceram Soc 57:394–397
Rostami V, Shao Y, Boyd A (2011) Durability of concrete pipes subjected to combined steam and carbonation curing. Constr Build Mater 25:3345–3355
Shao Y, Mirza MS, Wu X (2006) CO2 Sequestration using calcium-silicate concrete. Can J Civ Eng 33:776–784
Mahoutian M, Shao Y, Mucci A, Fournier B (2015) Carbonation and hydration behaviour of EAF and BOF steel slag binder. Mater Struct 48:3075–3085
World Steel Association (2014) Crude steel production data 2014–2015. Brussels, Belgium, p 2. https://www.worldsteel.org/dms/internetDocumentList/steel-stats/2015/Crude-steel-production-Jan-Dec-2015-vs-2014_/document/Crude%20steel%20production%20Jan-Dec%202015%20vs%202014.pdf
Chung F (1974) Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J Appl Crystallogr 7:519–525
Uibu M, Kuusik R, Andreas L, Kirsimae K (2011) The CO2-binding by Ca-Mg-silicates in direct aqueos carbonation of oil shale ash and steel slag. Energy Procedia 4:925–932
Taylor HFW (1997) Cement chemistry, 2nd edn. Thomas Telford Publishing, London
Shi C (2004) Steel slag-its production, processing, characteristics, and cementitious properties. J Mater Civ Eng 16:230–236
Madloola N, Saidura R, Hossaina M, Rahim N (2011) A critical review on energy use and savings in the cement industries. Renew Sustain Energy Rev 15:2042–2060
Torgal F, Cabeza LF, Labrincha J, Magalhaes A (2014) Eco-efficient construction and building materials-Life cycle assessment, eco-labeling and case studies. Woodhead Publishing, Cambridge
U.S. Geological Survey (2014) Mineral commodity summaries 2014. U.S. Geological Survey, p 196
Acknowledgments
Financial support by Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) is gratefully acknowledged. The authors are also thankful to Quebec Rio Tinto Iron & Titanium (RTIT) for ladle slag and to Quebec RLF Canada for waste glass.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahoutian, M., Ghouleh, Z. & Shao, Y. Synthesis of waste-based carbonation cement. Mater Struct 49, 4679–4690 (2016). https://doi.org/10.1617/s11527-016-0816-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-016-0816-6