Abstract
Objectives
We previously estimated the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies following the first pandemic wave at 2.23% in Québec, Canada. Following the much bigger second wave in fall 2020 and early 2021, we estimated the seroprevalence of anti-SARS-CoV-2 in Québec during the first months of 2021.
Methods
Blood samples from regular, asymptomatic (for ≥ 14 days) donors were collected between January 25, 2021 and March 11, 2021. Anti-SARS-CoV-2 seropositivity was assessed using an enzyme-linked immunosorbent assay that captures antibodies directed against the receptor binding domain of the SARS-CoV-2 spike (and hence cannot discriminate between infection- and vaccine-induced seropositivity). Seroprevalence estimates were adjusted for regional distribution, age, and sex.
Results
Samples from 7924 eligible donors were analyzed, including 620 (7.8%) vaccinated donors and 7046 (88.9%) unvaccinated donors (vaccination status unknown for 258 (3.3%) donors). Overall, median age was 51 years; 46.4% of donors were female. The adjusted seroprevalence was 10.5% (95% CI = 9.7–11.3) in the unvaccinated population and 14.7% (95% CI = 13.8–15.6) in the overall population. Seroprevalence gradually decreased with age and was higher among donors who self-identified as having a racial/ethnic background other than white, both in the overall and in the unvaccinated populations.
Conclusion
The seroprevalence of SARS-CoV-2 antibodies significantly increased in Québec since spring 2020, with younger persons and ethnic minorities being disproportionately affected. When compared with the cumulative incidence rate reported by public health authorities (i.e., 3.3% as of March 11, 2021), these results suggest that a substantial proportion of infections remain undetected despite improvements in access to COVID-19 testing.
Résumé
Objectifs
Lors d’une première étude, nous avons estimé la séroprévalence des anticorps contre le syndrome respiratoire aigu sévère coronavirus 2 (SRAS-CoV-2) après la première vague pandémique à 2,23 % au Québec, Canada. Cette seconde étude estime la séroprévalence de l’anti-SRAS-CoV-2 au Québec lors de la deuxième vague pandémique.
Méthodes
Des échantillons de donneurs de sang asymptomatiques (≥ 14 jours) ont été prélevés entre le 25 janvier et le 11 mars 2021. La séropositivité a été évaluée à l’aide d’un dosage immuno-enzymatique qui capture les anticorps dirigés contre la protéine Spike du récepteur de domaine de liaison du SARS-CoV-2 (et ne peut donc distinguer l’immunité induite par l’infection et la vaccination). La séroprévalence a été ajustée en fonction de l’âge et du sexe par région.
Résultats
Des échantillons de 7 924 donneurs ont été analysés, dont 620 (7,8 %) étaient vaccinés et 7 046 (88,9 %) étaient non vaccinés (statut vaccinal inconnu pour 258 (3,3 %) donneurs). Dans l’ensemble, l’âge médian était de 51 ans et 46,4 % des donneurs étaient des femmes. La séroprévalence ajustée était de 10,5 % (IC 95 % = 9,7 à 11,3) dans la population non vaccinée et de 14,7 % (IC 95 % = 13,8 à 15,6) dans la population globale. La séroprévalence diminuait progressivement avec l’âge et était plus élevée chez les donneurs d’origine ethnique autre que blanche.
Conclusion
La séroprévalence anti-SRAS-CoV-2 a considérablement augmenté au Québec depuis le printemps 2020, les personnes plus jeunes et les minorités ethniques étant plus touchées. Comparés au taux d’incidence cumulatif signalé par la santé publique (c.-à-d. 3,3 % au 11 mars 2021), ces résultats suggèrent qu’une proportion importante d’infections reste non détectée.
Similar content being viewed by others
Introduction
Early in the pandemic, public health authorities from developed countries implemented RT-PCR–based screening tests to identify cases of coronavirus disease-19 (COVID-19), detect community outbreaks, and limit disease spread. Despite the unprecedented scale of these efforts, public health surveillance data may be prone to biases, including the limited testing of most asymptomatic individuals (Padula, 2020), the (likely) underreporting of symptomatic cases, and limited or unequal access to testing in some areas (Dryden-Peterson et al., 2021; Lieberman-Cribbin et al., 2020; Souch & Cossman, 2021). As a result, public health data on documented infections likely underestimate the incidence of COVID-19. Reliable data on the cumulative incidence of COVID-19, overall and in specific regions or subgroups, are necessary to inform containment policies and validate dynamic models that aim to predict the evolution of the pandemic.
Serosurveys can address some of these limitations through the identification of individuals with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibodies, which can generally be detected within 14 days post-symptom onset (Long et al., 2020; Prévost et al., 2020; Seow et al., 2020). Given their expertise and the nature of their routine operations, blood banks are particularly well positioned to perform large-scale serosurveys and have played a central role in several such studies (O’Brien et al., 2021). Further, the World Health Organization has recognized blood donors as a reliable data source to study the seroprevalence of SARS-CoV-2 antibodies (World Health Organization, 2020).
We previously estimated the seroprevalence of anti-SARS-CoV-2 after the first pandemic wave at 2.23% in Québec, Canada, based on samples from regular blood donors collected in May and June 2020 (i.e., the “phase 1 serosurvey”) (Lewin et al., 2021). This rate was approximately four times as high as the cumulative incidence reported by public health authorities through RT-PCR–based screening, suggesting a substantial proportion of cases go undetected. Québec has since experienced a significant second wave of COVID-19 cases between September 2020 and March 2021. Therefore, an update on the seroprevalence of SARS-CoV-2 antibodies was needed. Using blood donors, we estimated the seroprevalence of SARS-CoV-2 antibodies in the Québec general population between January 2021 and March 2021.
Methods
Study participants
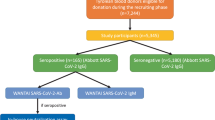
The methodology was similar to that used in the phase 1 serosurvey (Lewin et al., 2021). Briefly, regular blood donors were included if they met the following criteria: (1) donated blood between January 25, 2021 and March 11, 2021—a period characterized by the vaccination of priority groups, including older persons and health care workers; (2) resided in Québec; (3) were free of COVID-19 symptoms in the preceding 14 days; and (4) met standard blood donation criteria. Blood samples were obtained from consenting blood donors who gave a regular blood donation at one of Héma-Québec’s permanent donation centres or mobile blood drives located in 14 out of the 18 health regions in Québec. This study was approved by the ethics research board of Héma-Québec.
Anti-SARS-CoV-2 Spike RBD ELISA
The study outcome was anti-SARS-CoV-2 seropositivity, which was determined using an in-house, enzyme-linked immunosorbent assay (ELISA) that captures antibodies targeting the receptor binding domain (RBD) of the SARS-CoV-2 spike protein (Perreault et al., 2020; Lewin et al., 2021). Of note, this assay cannot discriminate between seropositivity due to a prior infection versus vaccination, since spike is the immunogen used in current vaccines. Therefore, seropositivity results were stratified by vaccination status (i.e., the “overall population” and “unvaccinated population”), which was obtained from the Système d’information pour la protection des maladies infectieuses registry (Ministère de la Santé et des Services sociaux, 2021).
Results were further stratified by sex, self-reported race/ethnicity, material deprivation index, and social deprivation index. Race/ethnicity was assessed given the compelling data supporting that people of colour have a higher risk of hospitalization and death due to COVID-19 (Centers for Disease Control and Prevention, 2021).
Seroreversion substudy
All donors who tested positive in the phase 1 serosurvey (n = 173) were contacted for phase 2 (i.e., the current study) to determine the proportion who seroreverted. For these patients, blood samples were collected ~ 7–10 months after the previous serological assessment.
Statistical analyses
Seroprevalence rates, along with Clopper-Pearson confidence intervals (CI), were adjusted for regional distribution, and age and sex in each region based on overall and region-specific 2011 census data. In the unvaccinated population, adjustment for seroreversion was done by adding to the observed seroprevalence the product of the proportion of donors who seroconverted in the seroreversion substudy and the seroprevalence in the phase 1 serosurvey. In the overall population, this adjustment was done only in unvaccinated individuals, given that none of the vaccinated individuals seroreverted.
Results
Samples from 7924 eligible donors were analyzed, including 620 (7.8%) who had received at least one vaccine dose (81 (1.0%) had received two doses) and 7046 (88.9%) who were unvaccinated at the time of the study; vaccination status was unknown for 258 (3.3%) donors. The Astra Zeneca, Moderna, and Pfizer vaccines were approved at the time of this analysis, and 93% of vaccinated donors had received the Pfizer vaccine. Median time since receipt of the last vaccine dose was 31 days for seropositive donors and 6 days for seronegative donors. In the overall population, median age was 51 years (interquartile range = 28 years) and 46.4% were female.
Before statistical adjustments, 1191 (15.0%) donors in the overall population, 685 (9.7%) donors in the unvaccinated population, and 476 (76.7%) donors in the vaccinated population had a seropositive test result (Table 1). The adjusted seroprevalence was 10.5% (95% CI = 9.7–11.3) in the unvaccinated population, 14.7% (95% CI = 13.8–15.6) in the overall population, and 76.8% (95% CI = 73.5–80.0) in the vaccinated population (Fig. 1 and Table S1).
Seroprevalence estimates adjusted for seroreversion
In total, 109 out of 173 (63.0%) donors with a seropositive test result in the phase 1 serosurvey provided informed consent and were included in the analysis of seroreversion. In agreement with previous studies showing a decrease of anti-spike levels over time (Anand et al., 2021; Perreault et al., 2020), 32 (29.4% (95% CI = 21.0–38.9)) had become seronegative (i.e., had seroreverted) 7–10 months after their initial test (Table S6). This proportion remained similar regardless of the age group considered. More than 277 days elapsed between the first blood sample collected (carried out during the phase 1 serosurvey) and the second serological test for a majority of individuals who seroreverted (55.6% (95% CI = 30.8–78.5)).
Considering this seroreversion rate, the adjusted seroprevalence would increase to 11.2% (95% CI = 10.4–11.9) for the unvaccinated population and 15.3% (95% CI = 14.4–16.2) for the overall population.
Seroprevalence estimates stratified by region
In the overall population, the greater area of Montreal-Laval (seroprevalence = 17.1% (95% CI = 15.3–18.8)), but not the surrounding urban areas (12.3% (95% CI = 10.5–14.2)), exhibited an above-average adjusted seroprevalence (Fig. 1); data on other regions are available in Table S1. Similar observations were made in the unvaccinated population (Fig. 1 and Table S1).
Regional seroprevalence in the unvaccinated population strongly correlated with cumulative incidence rates reported by Québec public health authorities (r = 0.83; Fig. 2). However, the ratio between the observed seroprevalence in the unvaccinated population and the cumulative incidence rate reported by public health authorities—an indicator of underreporting hereinafter referred to as the “undiagnosed ratio”—varied widely among regions (range: 2.3 (Lanaudière) to 7.01 (Outaouais)). Furthermore, the undiagnosed ratio was inversely correlated with the cumulative incidence reported by public health authorities (Fig. 3).
Seroprevalence estimates stratified by sex, age, and self-reported race/ethnicity
In the overall population, the adjusted seroprevalence was significantly higher among females (seroprevalence = 16.9% (95% CI = 15.6–18.3)) than among males (12.5% (95% CI = 11.3–13.7)), as shown by the non-overlap of 95% CIs (Fig. 1 and Table S2). However, in the unvaccinated population, rates were similar between females (10.9% (95% CI = 9.7–12.0)) and males (10.2% (95% CI = 9.1–11.3); Fig. 1 and Table S2). Seroprevalence gradually decreased with age, both in the overall and unvaccinated populations (Fig. 1 and Tables S3-S4). Donors who self-identified as having a racial/ethnic background other than white had a seroprevalence nearly twice as high as those who self-identified as white (Fig. 1 and Table S5), both in the unvaccinated (white: 9.5% (95% CI = 8.7–10.4); other: 19.3% (95% CI = 16.0–22.5)) and overall populations (white: 13.9% (95% CI = 13.0–14.9); other: 22.1% (95% CI = 18.7–25.4); see Table S5 for data on individual races/ethnicities). This difference corresponds to a relative risk (RR) of 2.01 (95% CI = 1.66–2.44) in the unvaccinated population and 1.48 (95% CI = 1.25–1.75) in the overall population. No discernable trend or statistically significant difference was observed when results were stratified by material or social deprivation index (Fig. 1).
Discussion
Between February 2021 and April 2021, the adjusted seroprevalence of SARS-CoV-2 antibodies, corrected for seroreversion, was estimated at 11.2% (95% CI = 10.4–11.9) in the unvaccinated population and 15.4% (95% CI = 14.5–16.3) in the overall population. Compared with the cumulative incidence rate reported by public health authorities (i.e., 3.3% as of March 11, 2021), the estimate in the unvaccinated population suggests that 2.4 COVID-19 cases may go undetected for every confirmed case reported to public health authorities. This ratio of undiagnosed to diagnosed cases (i.e., 2.4:1) is largely similar to that observed in our previous assessment of seroprevalence in May and June 2020 (2.2% vs. 0.6%; a ~ 3:1 ratio) (Lewin et al., 2021). Taken together, and consistent with other published results (Byambasuren et al., 2021), our data suggest that a substantial proportion of SARS-CoV-2 infections remain undetected, despite notable improvements in access to testing since the early phase of the pandemic.
In phase 2 of the Tang et al. study (October 2020–March 2021), 16.0% of contacted panel members provided dried blood spots and contributed to the final analysis. Seropositivity was defined by the presence of anti-spike, anti-RBD, or anti-nucleocapsid antibodies, as assessed by ELISA. The education-adjusted seroprevalence was 6.4% in Québec (Tang et al., 2022). While seemingly lower, this estimate is not comparable with that of the current study since this study did not adjust for age and sex, was conducted over a 6-month period (vs. < 3 months in the current study), used a different definition of seropositivity, and was limited by a low response rate.
The population analyzed in this study was sufficiently large to derive estimates that may be generalized to the overall Québec population. Estimates were adjusted for age, sex, and regional distribution, which should control for the modest differences between the characteristics of our sample and those of Québec’s general population. However, a caveat of our analysis is the apparent underrepresentation of visible minorities in the study sample (current study: 7.3%, 2016 census: 13.0%) (Statistics Canada, 2017), which likely underestimated seroprevalence given that these groups were disproportionately affected by COVID-19 (Kalish et al., 2021; Saeed et al., 2021). Of note, nearly 90% of participants were not vaccinated, which was expected given that vaccine rollout only started in April 2021 for individuals younger than 70 years who were not health care workers. The rate of seroconversion appeared suboptimal in the vaccinated population (i.e., 76.7%), likely because time since receipt of the vaccine dose (median = 31 days for seropositive and 6 days for seronegative donors) did not enable the humoral response to peak (Table 2), which can take up to 29 days according to vaccine immunogenicity studies (Jackson et al., 2020; Walsh et al., 2020).
Interestingly, regions with relatively low cumulative incidence rates (as reported by public health authorities) tended to exhibit the highest undiagnosed ratios, suggesting a greater proportion of undiagnosed infections. Several factors, such as limited access to testing, lack of disease awareness, and fear of stigma, may drive this apparent underreporting in regions with low incidence rates (Earnshaw et al., 2020; McElfish et al., 2021; Sotgiu & Dobler, 2020).
Seroprevalence was similar in unvaccinated individuals of both sexes. When vaccinated individuals were included, seroprevalence was higher among females than among males. This observation may in part be driven by the overrepresentation of women among essential workers (e.g., health care workers), who were prioritized for vaccine rollout. Additionally, in one study, COVID-19 vaccines seemingly elicited a stronger and more sustained humoral response in women than in men (Shrotri et al., 2021), as observed for many vaccines in other disease areas (Fischinger et al., 2019). However, no statistical test was performed between the groups in that study (Shrotri et al., 2021), so that this potential explanation remains highly uncertain.
The higher seroprevalence observed in younger donors is consistent with the high incidence of confirmed COVID-19 cases reported by public health authorities (Institut national de santé publique du Québec, 2021). Recent evidence suggests that younger individuals are less adherent to COVID-19 preventive measures (World Health Organization, 2021), which might partially explain this observation. Young adults generally have more contacts than older ones, which also increases their risk of infection. Occupational exposure might also contribute to this finding, since the steepest decline in age-stratified seroprevalence occurred between donors aged 40–59 years and those aged 60–69 years, which generally corresponds to retirement age. Of note, a similar decline can be observed in cumulative incidence rates reported by public health authorities (Institut national de santé publique du Québec, 2021).
Seroprevalence among non-white unvaccinated donors was almost twice as high as that among white donors, in line with findings from previous serosurveys that the pandemic had a disproportionate toll on ethnic minorities (Kalish et al., 2021; Saeed et al., 2021). Additional outreach efforts may thus be necessary to limit the spread of COVID-19 in communities affected by linguistic, occupational, and cultural barriers.
Several studies have shown that the levels of SARS-CoV-2–specific antibodies begin to decline 1 to 4 months following symptom onset (Anand et al., 2021; Beaudoin-Bussières et al., 2020; Masiá et al., 2021; Perreault et al., 2020; Prévost et al., 2020; Seow et al., 2020; Zhang et al., 2020), so that some previously infected donors might have transitioned from a seropositive to a seronegative state (i.e., seroreversion) since the beginning of the pandemic. The analysis of seroreversion showed that 32 out of 109 (29.4%) consenting donors had seroreverted 7–10 months after their last serological test.
Limitations
The current study has limitations. First, the anti-RBD ELISA cannot discriminate between infection- and vaccine-induced seropositivity. Although seroprevalence was stratified by vaccination status to mitigate this limitation, seroprevalence in the unvaccinated population is likely underestimated. Second, per eligibility criteria, symptomatic donors were excluded from this study, thereby underestimating the seroprevalence of SARS-CoV-2 antibodies and the undiagnosed ratios. Our results may therefore be viewed as a complement to the cumulative incidence rate reported by public health authorities, which is more likely to capture symptomatic cases. Third, estimates could not be adjusted for race/ethnicity because of the relatively small sample sizes of some groups, as well as discrepancies in racial categories between census and internal data, thereby potentially underestimating seroprevalence and undiagnosed ratios. Fourth, asymptomatic donors with an active infection might have been classified as seronegative if the sample was collected before the onset of a robust humoral response. Fifth, some low-density areas (i.e., Nord-du-Québec, Nunavik, and Terres Cries de la Baie James) could not be sampled owing to the absence of centres or mobile drives in these regions at the time of study participation. Last, despite the high sensitivity (98.9%) and specificity (98.5%) of the anti-RBD ELISA used in this study (Perreault et al., 2020), some donors might have been misclassified. Had we adjusted for the assay’s sensitivity and specificity using the Rogan-Gladen estimator (Rogan & Gladen, 1978), seroprevalence (95% CI) would have been 13.6% (12.6–14.5) in the overall population (vs. 14.7% without such adjustments) and 9.2% (8.4–10.1) in the unvaccinated population (vs. 10.5% without such adjustments).
Conclusion
In this analysis of nearly 8000 blood donors in Québec, the seroprevalence of SARS-CoV-2 antibodies was estimated at 10.5% in the unvaccinated population and 11.2% after accounting for seroreversion. These figures are three times as high as the cumulative incidence rates reported by public health authorities, suggesting that a substantial proportion of cases remain undetected.
Contributions to knowledge
What does this study add to existing knowledge?
-
This study suggests that the seroprevalence of SARS-CoV-2 antibodies significantly increased between February and April 2021 in Québec, Canada, and that up to 75% of cases may not be detected by public health surveillance efforts.
-
The results also confirm that younger persons and ethnic minorities were disproportionately infected by COVID-19.
What are the key implications for public health interventions, practice, or policy?
-
Additional public health measures may be warranted to increase diagnostic rates and alleviate the burden of the pandemic on vulnerable groups, such as younger persons and people of colour.
Availability of data and material
Data and material are available upon request.
Code availability
Code is available upon request.
References
Anand, S. P., Prévost, J., Richard, J., Perreault, J., Tremblay, T., Drouin, M., Fournier, M.-J., Lewin, A., Bazin, R., & Finzi, A. (2021). High-throughput detection of antibodies targeting the SARS-CoV-2 spike in longitudinal convalescent plasma samples. Transfusion, 61(5), 1377–1382. https://doi.org/10.1111/trf.16318
Beaudoin-Bussières, G., Laumaea, A., Anand, S. P., Prévost, J., Gasser, R., Goyette, G., Medjahed, H., Perreault, J., Tremblay, T., Lewin, A., Gokool, L., Morrisseau, C., Bégin, P., Tremblay, C., Martel-Laferrière, V., Kaufmann, D. E., Richard, J., Bazin, R., & Finzi, A. (2020). Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. MBio, 11(5), e02590–e02520. https://doi.org/10.1128/mBio.02590-20
Byambasuren, O., Dobler, C. C., Bell, K., Rojas, D. P., Clark, J., McLaws, M.-L., & Glasziou, P. (2021). Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: systematic review. PLoS One, 16(4), e0248946. https://doi.org/10.1371/journal.pone.0248946
Centers for Disease Control and Prevention. (2021). Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Accessed August 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
Données Québec. (2020). Estimations et projections de population comparables. Accessed September 9, 2021. https://www.donneesquebec.ca/recherche/dataset/estimations-et-projections-de-population-comparables/resource/05c3c53d-db97-4bc3-8221-d8af47b4b93e
Dryden-Peterson, S., Velásquez, G. E., Stopka, T. J., Davey, S., Lockman, S., & Ojikutu, B. O. (2021). Disparities in SARS-CoV-2 testing in Massachusetts during the COVID-19 pandemic. JAMA Network Open, 4(2), e2037067. https://doi.org/10.1001/jamanetworkopen.2020.37067
Earnshaw, V. A., Brousseau, N. M., Hill, E. C., Kalichman, S. C., Eaton, L. A., & Fox, A. B. (2020). Anticipated stigma, stereotypes, and COVID-19 testing. Stigma and Health, 5(4), 390–393. https://doi.org/10.1037/sah0000255
Fischinger, S., Boudreau, C. M., Butler, A. L, Streeck, H., & Alter, G. (2019). Sex differences in vaccine-induced humoral immunity. Seminars in Immunopathology, 41(2), 239-249. doi:https://doi.org/10.1007/s00281-018-0726-5
Institut national de santé publique du Québec. (2021). Données COVID-19 par âge et sexe au Québec. Accessed August 12, 2021. https://www.inspq.qc.ca/covid-19/donnees/age-sexe
Jackson, L. A., Anderson, E. J., Rouphael, N. G., Roberts, P. C., Makhene, M., Coler, R. N., McCullough, M. P., Chappell, J. D., Denison, M. R., Stevens, L. J., et al. (2020). An mRNA vaccine against SARS-CoV-2—preliminary report. The New England Journal of Medicine, 383(20), 1920–1931.
Kalish, H., Klumpp-Thomas, C., Hunsberger, S., Baus, H. A., Fay, M. P., Siripong, N., Wang, J., Hicks, J., Mehalko, J., Travers, J., Drew, M., Pauly, K., Spathies, J., Ngo, T., Adusei, K. M., Karkanitsa, M., Croker, J. A., Li, Y., Graubard, B. I., et al. (2021). Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Science Translational Medicine, 13(601), eabh3826. https://doi.org/10.1126/scitranslmed.abh3826
Lewin, A., Therrien, R., De Serres, G., Grégoire, Y., Perreault, J., Drouin, M., Fournier, M. J., Tremblay, T., Beaudoin, J., Beaudoin-Bussières, G., Prévost, J., Gendron-Lepage, G., Finzi, A., Bernier, F., Bazin, R., Germain, M., & Delage, G. (2021). SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Canadian Journal of Public Health, 112(4), 576–586. https://doi.org/10.17269/s41997-021-00531-6
Lieberman-Cribbin, W., Tuminello, S., Flores, R. M., & Taioli, E. (2020). Disparities in COVID-19 testing and positivity in New York City. American Journal of Preventive Medicine, 59(3), 326–332. https://doi.org/10.1016/j.amepre.2020.06.005
Long, Q. X., Liu, B. Z., Deng, H. J., Wu, G. C., Deng, K., Chen, Y. K., Liao, P., Qiu, J. F., Lin, Y., Cai, X. F., Wang, D. Q., Hu, Y., Ren, J. H., Tang, N., Xu, Y. Y., Yu, L. H., Mo, Z., Gong, F., Zhang, X. I., et al. (2020). Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine, 26(6), 845–848. https://doi.org/10.1038/s41591-020-0897-1
Masiá, M., Fernández-González, M., Telenti, G., Agulló, V., García, J. A., Padilla, S., García-Abellán, J., Galiana, A., Gonzalo-Jiménez, N., & Gutiérrez, F. (2021). Durable antibody response one year after hospitalization for COVID-19: a longitudinal cohort study. Journal of Autoimmunity, 123, 102703. https://doi.org/10.1016/j.jaut.2021.102703
McElfish, P. A., Purvis, R., James, L. P., Willis, D. E., & Andersen, J. A. (2021). Perceived barriers to COVID-19 testing. International Journal of Environmental Research and Public Health, 18(5), 2278. https://doi.org/10.3390/ijerph18052278
Ministère de la Santé et des Services sociaux. (2021). Registre de vaccination du Québec. Accessed August 12, 2021. https://msss.gouv.qc.ca/professionnels/vaccination/registre-vaccination/deploiement/#implantation-module
O’Brien, S. F., Lieshout-Krikke, R. W., Lewin, A., Erikstrup, C., Steele, W. R., Uzicanin, S., & Custer, B. (2021). Research initiatives of blood services worldwide in response to the Covid-19 pandemic. Vox Sanguinis, 116(3), 296–304. https://doi.org/10.1111/vox.12995
Padula, W. V. (2020). Why only test symptomatic patients? Consider random screening for COVID-19. Applied Health Economics and Health Policy, 18(3), 333–334. https://doi.org/10.1007/s40258-020-00579-4
Perreault, J., Tremblay, T., Fournier, M. J., Drouin, M., Beaudoin-Bussières, G., Prévost, J., Lewin, A., Bégin, P., Finzi, A., & Bazin, R. (2020). Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood, 136(22), 2588–2591. https://doi.org/10.1182/blood.2020008367
Prévost, J., Gasser, R., Beaudoin-Bussières, G., Richard, J., Duerr, R., Laumaea, A., Anand, S. P., Goyette, G., Benlarbi, M., Ding, S., Medjahed, H., Lewin, A., Perreault, J., Tremblay, T., Gendron-Lepage, G., Gauthier, N., Carrier, M., Marcoux, D., Piché, A., et al. (2020). Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Reports. Medicine, 1(7), 100126. https://doi.org/10.1016/j.xcrm.2020.100126
Rogan, W. J., & Gladen, B. (1978). Estimating prevalence from the results of a screening test. American Journal of Epidemiology, 107(1), 71–76. https://doi.org/10.1093/oxfordjournals.aje.a112510
Saeed, S., Drews, S. J., Pambrun, C., Yi, Q. L., Osmond, L., & O’Brien, S. F. (2021). SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion, 61(3), 862–872. https://doi.org/10.1111/trf.16296
Seow, J., Graham, C., Merrick, B., Acors, S., Pickering, S., Steel, K. J. A., Hemmings, O., O’Byrne, A., Kouphou, N., Galao, R. P., Betancor, G., Wilson, H. D., Signell, A. W., Winstone, H., Kerridge, C., Huettner, I., Jimenez-Guardeño, J. M., Lista, M. J., Temperton, N., et al. (2020). Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiology, 5(12), 1598–1607. https://doi.org/10.1038/s41564-020-00813-8
Shrotri, M., Navaratnam, A., Nguyen, V., Byrne, T., Geismar, C., Fragaszy, E., Beale, S., Fong, W., Patel, P., Kovar, J., Hayward, A., & Aldridge, R. (2021). Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet (London, England), 398(10298), 385–387. https://doi.org/10.1016/S0140-6736(21)01642-1
Sotgiu, G., & Dobler, C. C. (2020). Social stigma in the time of coronavirus disease 2019. The European Respiratory Journal, 56(2), 2002461. https://doi.org/10.1183/13993003.02461-2020
Souch, J. M., & Cossman, J. S. (2021). A commentary on rural-urban disparities in COVID-19 testing rates per 100,000 and risk factors. The Journal of Rural Health : Official Journal of the American Rural Health Association and the National Rural Health Care Association, 37(1), 188–190. https://doi.org/10.1111/jrh.12450
Statistics Canada. (2017). Quebec [province] and Canada [country] (table). Census profile. 2016 Census. Accessed September 9, 2021. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang = E
Tang, X., Sharma, A., Pasic, M., Brown, P., Colwill, K., Gelband, H., Birnboim, H. C., Nagelkerke, N., Bogoch, I. I., Bansal, A., Newcombe, L., Slater, J., Rodriguez, P. S., Huang, G., Hang Fu, S., Meh, C., Wu, D. C., Kaul, R., Langlois, M-A., Morawski, E., Hollander, A., Eliopoulos, D., Aloi, B., Lam, T., Abe, K. T., Rathod, B., Fazel-Zarandi, M., Wang, J., Iskilova, M., Pasculescu, A., Caldwell, L., Barrios-Rodiles, M., Mohammed-Ali, Z., Vas, N., Raman Santhanam, D., Rin Cho, E., Qu, K., Jha, S., Jha, V., Suraweera, W., Malhotra, V., Mastali, K., Wen, R., Sinha, S., Reid, A., Gingras, A-C., Chakraborty, P., Slutsky, A. S., Jha, P., for the Ab-C Study Investigators. (2022). Assessment of SARS-CoV-2 Seropositivity During the First and Second Viral Waves in 2020 and 2021 Among Canadian Adults. JAMA Network Open, 5(2):e2146798. https://doi.org/10.1001/jamanetworkopen.2021.46798
Walsh, E. E., Frenck Jr., R. W., Falsey, A. R., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Neuzil, K., Mulligan, M. J., & Bailey, R. (2020). Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New England Journal of Medicine, 383(25), 2439–2450.
World Health Organization. (2020). Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection. Accessed August 12, 2021. https://apps.who.int/iris/bitstream/handle/10665/331656/WHO-2019-nCoV-Seroepidemiology-2020.1-eng.pdf?sequence = 1&isAllowed = y
World Health Organization. (2021). Young People and COVID-19: Behavioural considerations for promoting safe behaviours. Accessed August 12, 2021. https://www.who.int/publications/i/item/978-92-4-002831-9
Zhang, G., Nie, S., Zhang, Z., & Zhang, Z. (2020). Longitudinal change of severe acute respiratory syndrome coronavirus 2 antibodies in patients with coronavirus disease 2019. The Journal of Infectious Diseases, 222(2), 183-188. doi:https://doi.org/10.1093/infdis/jiaa229
Acknowledgements
The authors are grateful to the blood donors who participated in this study and the Héma-Québec team involved in this project. Medical writing assistance was provided by Samuel Rochette and Jean-François Leblanc, who are employees of Héma-Québec.
The data presented in the current manuscript were previously disseminated in a government report. All major contributors of this work were contacted and agree to this publication.
Funding
This work was funded by the Ministère de la Santé et des Services Sociaux du Québec. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AL, GD, YG, GDS, RB, MG, CR: conceptualization; formal analysis; methodology; writing original draft. AB: project coordination; manuscript revision. AL, GD, GDS, AF, CR, MG, RB, GG: methodology; reagents; writing—review and editing. JP, TT, YG, MD, MJF, JB: laboratory analysis; results interpretation. Every author has read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by Héma-Québec’s Research Ethics Committee.
Consent to participate
The participants consented to participate in this study.
Consent for publication
Publication was foreseen in the acceptance of participation.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Lewin, A., De Serres, G., Grégoire, Y. et al. Seroprevalence of SARS-CoV-2 antibodies among blood donors in Québec: an update from a serial cross-sectional study. Can J Public Health 113, 385–393 (2022). https://doi.org/10.17269/s41997-022-00622-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.17269/s41997-022-00622-y