Artificial intelligence-based myocardial texture analysis in etiological differentiation of left ventricular hypertrophy
Introduction
Left ventricular hypertrophy (LVH) is a common clinical finding related to adverse cardiovascular outcomes, such as myocardial ischemia, arrythmia, congestive heart failure and sudden cardiac death (1,2). Main etiologies are hypertension, hypertrophic cardiomyopathy (HCM), aortic valve stenosis, uremia, and cardiac amyloidosis. Other less common causes include myocarditis, cardiac sarcoidosis, and a few genetic disorders such as Anderson-Fabry disease, Danon disease, mitochondrial cardiomyopathies and more. Transthoracic echocardiography (TTE) plays an important role in LVH diagnosis. It not only provides a comprehensive evaluation of LV morphology and functions, but also detects concurrent structural abnormalities. These information may lead to a definite etiology in a subset of patients, but not necessarily enough in the others for lack of specificity (3,4). Myocardial texture is largely neglected in conventional TTE, because it is usually too subtle, non-specific, and difficult to rate or quantify based on human visual observation. Artificial intelligence (AI) has been developing rapidly in recent years. Previous researchers have reported the application of radiomics and AI-based algorithms from cardiac magnetic imaging (CMR), cardiac computed tomography (CT) and echocardiography data in several myocardial diseases (5-9). However, none has been devoted to multiple LVH etiology differentiation based on echocardiography. Compared with CMR and cardiac CT, TTE is more convenient, cost-effective, and commonly available in community hospitals. We hypothesized that the disparities in LVH histological changes from variant etiologies would lead to different myocardial textures in the echocardiographic image, and the computer might be able to recognize them by quantification and algorithms. Specifically, this study aimed to investigate myocardial texture in hypertensive heart disease (HHD), HCM and uremic cardiomyopathy (UCM) through radiomics, and test the features with an AI-based classifier. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4891).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Research Ethics Committee of Shanghai East Hospital (No. 2020-028) and individual consent for this retrospective analysis was waived.
Study population
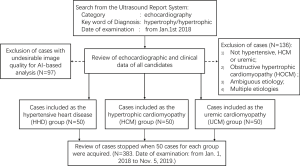
Study population were recruited retrospectively as shown in the flow chart in Figure 1. Case collection was initiated from the Ultrasound Report System of Shanghai East Hospital by searching the key word “hypertrophy” or “hypertrophic”. The date of examination was set from January 1, 2018, with an open end of time-point depending on the number of recruited cases. Next, echocardiographic, and clinical data were carefully reviewed for all the candidates consecutively, until 50 cases for each group were obtained. LVH was defined as left ventricular mass index (LVMI) >115 g/m2 in male, LVMI >95 g/m2 in female, and the greatest left ventricular (LV) wall thickness >13 mm (10). HHD was diagnosed for LVH patients with a history of arterial hypertension, and without a familial history of HCM or other abnormal loading conditions. HCM was diagnosed as the greatest LV wall thickness ≥15 mm that is not explained solely by loading conditions (11). Of note, obstructive HCM (HOCM, diagnosed as pressure gradient ≥30 mmHg) was eliminated from the study to avoid mixture. UCM was defined as LVH presentation in patients with chronic end-stage renal disease (ESRD), with the glomerular filtration rate (GFR) <15 mL/min/1.73 m2. Exclusion criteria include ambiguous etiologies, multiple etiologies (except UCM with hypertension), coronary heart disease, aortic valve stenosis, moderate or severe valvular regurgitation, congenital heart disease, cardiac amyloidosis, diabetes, athletic hearts, and cases with inadequate image qualities. UCM with hypertension were not excluded because all the UCM group members had arterial hypertension secondary to chronic renal failure.
Echocardiography
TTE had all been performed following the 2015 version of Recommendations of cardiac quantification in adults by the American Society of Echocardiography (ASE) (10). Multiple operators were involved, including novice and experienced echocardiographers. Ultrasound equipment used were GE Vivid E9 (transducer M5S), GE Vivid7 (transducer M4S), Philips IE33 (transducer S5-1) and Philips EPIQ7C (transducer S5-1 and X5-1) systems. Standard apical four-chamber (A4ch) view of the end-diastolic frame was retrieved for analysis.
Extraction of myocardial texture features
Firstly, a well visualized region of the interventricular septum (IVS) was manually outlined as the region of interest (ROI) by an experienced echocardiographer as shown in Figure 2. The mask image of the ROI was obtained through binarization processing. Then the texture features were extracted, including first-order statistics and gray level co-occurrence matrix (GLCM) features.

In this research, main first-order statistics features were mean (IMean), median (IMedia), standard deviation (Std), coefficient of variation (CoV), skewness (Skew), kurtosis (Kurt), histogram entropy (EtHis) and brightness entropy (EtBrt) of the pixel grayscales within the ROI. More details are available in Appendix 1.
The second category GLCM features were aimed to describe the texture by analyzing spatial correlations of the grayscale. Unlike first-order statistics features, GLCM not only focuses on overall grayscale amplitudes of the pixels, but also studies their spatial correlation characteristics (12). In GLCM analysis, the 256 levels of grayscale were simplified to eight to reduce computation, resulting in 8×8 grayscale cooccurrence matrix G(i,j) (I = 1,2, …, 8; j = 1,2, …, 8). Then the matrix of probability p(i,j) was obtained through normalization of G(i,j). The distance (d) was set as 1, 2, 3, …, 15 pixels, and the direction θ was 0, 45, 90 and 135 degrees. Then the texture features of distance d in four directions were averaged and regarded as the result.
There were four types of GLCM features used in our research—energy (E), contrast (Cont), entropy (Et) and homogeneity (Hm). Heterogeneous and rough texture presents high values of energy, contrast and entropy, and a low value of homogeneity. Each type contains 15 values (d = 1, 2, 3, …, 15 pixels), making a total of 60 GLCM features—E1 to E15, Cont1 to Cont15, Et1 to Et15, and Hm1 to Hm15. The interested reader can find more information, including mathematical equations in Appendix 1.
Intraobserver and interobserver variability was tested via repeated ROI drawing by the same echocardiographer, and another echocardiographer who was a novice.
Statistical analysis
Statistical analyses were performed to select potentially useful textures features for differentiation. Continuous variables were described as mean ± SD. Comparisons of continuous variables were performed with one-way ANOVA among three groups. Between any two groups, unpaired t test was used for normal distributed parameters, and KW test for non-normal distributed parameters. P value <0.05 was regarded as statistically significant. The classification threshold for each significant parameter was determined when Youden index (YI) was the highest, and diagnostic sensitivity, specificity, accuracy, and areas under the receiver operating characteristic curve (AUC) were calculated. Next, interobserver and intraobserver variability were tested using intra-class correlation coefficient (ICC). Features with AUCs >0.70 and ICC >0.50 were then considered as candidates for the subsequent procedure.
Validation of classification
The selected texture features above were tested for classification by supportive vector machine (SVM), a classifier using supervised learning. Radial basis function (RBF) was applied, and dataset was divided as 6:4 for training and validation. Sensitivity, specificity, accuracy, YI, and AUC were generated for classification of each group from the other two, and between any two groups.
Results
Patient characteristics and echocardiography
Patient demography and echocardiographic parameters are listed in Table 1. The HCM group showed the greatest IVS thickness, greatest IVS/LV posterior wall (LVPW) thickness ratio, and smallest LV end-diastolic diameter (LVEDd). UCM group had the thickest LVPW, largest LVEDd and lowest LV ejection fraction (LVEF). The P values by one-way ANOVA were all <0.05 for LV quantification, except E/e’ ratio. However, the UCM group showed a higher E/e’ ratio level than HHD, implying poorer LV diastolic function. Of note, atrial fibrillation (AF) cases were excluded in the E/e’ analysis.

Full table
Statistics of texture features
Among the 77 texture features, EtHis, EtBrt, Std, CoV, Skew, Cont7, E11, Hm5 and Et3 showed relatively better statistical significance, as listed in Table 2. HCM appeared significantly more homogeneous LV wall than HHD and UCM (all nine features: P≤0.005). HHD was more homogeneous than UCM, however, significant difference was only yielded in EtBrt and CoV (P=0.011 and P=0.008). Accordingly, AUCs of EtBrt and CoV were also larger than the others, as stated in Table 3 (HCM vs. other groups: AUCEtBrt =0.87, AUCCoV =0.87; HHD vs. UCM: AUCEtBrt =0.65, AUCCoV =0.66). EtHis and Std also showed relatively higher AUC (>0.70 for HCM vs. other groups). More details about the cutoff values, sensitivity, specificity, accuracy, YI and AUCs can be found in Appendix 1.

Full table

Full table
Interobserver and intraobserver variability were assessed with ICC and shown in Table 4. EtHis, E11 and Et3 presented undesirable consistency (interobserver ICC <0.50) and were excluded in the subsequent experiment.

Full table
Classification by SVM
EtBrt, Std and CoV were remained eventually for validation of classification. The AUC for HHD vs. HCM and UCM reached 0.70, which was markedly improved than any individual texture feature. The AUC for HHD vs. HCM, and HHD vs. UCM were also slightly improved (0.91 and 0.68). Details of diagnostic accuracy, sensitivity, specificity, and AUC can be found in Table 5.

Full table
Discussion
LVH is a common outcome in several clinical conditions, among which hypertension, HCM and chronic renal failure are some of the most common etiologies. TTE can evaluate cardiac morphology and function in LVH, which may lead to a definite cause in some cases. For example, a highly asymmetrical pattern of septal hypertrophy or LV apical hypertrophy suggests HCM (11). However, there are still large numbers of nonspecific cases. HCM may also present symmetrical hypertrophy, mimicking HHD. On the contrary, HHD may manifest somewhat asymmetrical hypertrophy as well, with the thickest portion at the basal septum, and even with an IVS/LVPW thickness ratio greater than 1.5 (12,13). The third group, UCM, is usually related to symmetrical LV hypertrophy, and the odds of LV dilation and LV ejection fraction (LVEF) decline is increased (14-16). Therefore, it could be challenging to reveal the real cause of LVH, especially for inexperienced echocardiographers. A comprehensive analysis of multiple imaging data and clinical information is required. Advanced imaging techniques like CMR may be needed, and even endomyocardial biopsy and genetic analysis, which are more costly and not commonly available.
Echocardiography is a fundamental, widely used imaging modality in cardiology. It is very effective in evaluating cardiac structures and functions. However, compared with CMR, TTE has very limited value in tissue characterization, the echogenicity and heterogeneity of myocardium is rarely described in echocardiography, because it is not specific in most cases, and very difficult to rate or quantify based on human visual observation. Hence, we proposed myocardial texture analysis by the computer to overcome the limitation, and hopefully improve TTE diagnostic power. According to our results, different LVH groups did appear different myocardial texture features in TTE, and they could be identified by the computer. Previous researchers have reported the feasibility and usefulness of myocardial texture analysis based on CMR, cardiac CT and echocardiography in several myocardial abnormalities, such as myocardial infarction, myocarditis, cardiac amyloidosis and HCM (5-9,17). In this study, we explored TTE myocardial texture analysis in differentiating three specific LVH entities—HHD, HCM and UCM. It turned out that EtBrt, Std and CoV had potentially good diagnostic power and reproducibility. The three parameters were then tested with the SVM model, a classifier using supervised learning. The classification results of EtBrt, Std and CoV as combined was overall improved than any individual feature. The AUC of distinguishing HHD from HCM reached 0.91 (highest AUC of individual features: 0.86), The AUC of separating HHD from the others reached 0.70 (highest AUC of individual features: 0.61). Classifier models based on AI may further improve diagnostic accuracy of the LVH etiology.
As the statistics showed, HCM presented the highest EtBrt, and lowest Std and CoV, which indicated the most homogeneous myocardial texture. UCM appeared the most heterogeneous, but was close to HHD. These findings could be correlated to the different histological changes. Echogenic features of tissues were determined by histological components and the unity of microstructure alignment (17). For instance, collagen tends to attenuate soundwaves more than cardiomyocyte. Microstructures are usually hyperechoic due to numerous acoustic boundaries (17-19), while water is echolucent. Previous literature stated that common pathological changes in LVH include cardiomyocyte hypertrophy and disarray, expansion of interstitial and perivascular collagen, and vascular thickening (20-22). We hypothesized that different etiologies may lead to different patterns of LV remodeling, with different levels of myocardial hyperplasia, myocardial disarray, and interstitial fibrosis. UCM develops an even more complex histological pattern by multiple factors, including chronic hypertension, anemia, hypervolemia, and mineral metabolic disorders, resulting in hypertrophy, focal dissolve of cardiomyocyte, interstitial fibrosis, myocardial calcification and oxalate deposition, which might explain the most heterogeneous texture (23).
There were a few limitations to our study. Firstly, it was a single center and retrospective study. The results were not generalizable. Inter-operator and inter-equipment variations of image quality could hardly be eliminated. Secondly, the diagnoses were largely established upon clinical and imaging data according to the latest guidelines and expert consensus. The use of histological or genetic evidence was lacking. Thirdly, most HCM cases in the study had an LV wall over 15 mm thick, and the average maximum thickness was significantly greater than the other two groups. It was possible that the difference in myocardial texture was more related to the severity of hypertrophy, rather than the etiology. The study was preliminary. However, the application of AI in echocardiography is promising. There have been studies on TTE view recognition, image quality assessment, myocardial motion analysis, diagnostic algorithm establishment and more. There may come a day that a robot can replace the human expert entirely, from automated ultrasound scanning to intellectual image interpretation.
Conclusions
The current study has preliminarily investigated the feasibility of AI-assisted myocardial texture analysis in distinguishing HHD, HCM and UCM based on TTE still images. It turned out that HCM had a markedly more homogenous texture than HHD and UCM. UCM appeared the most heterogeneous texture, but was close to HHD. Within all the parameters, EtBrt and CoV showed the best diagnostic efficacy. Moreover, SVM model using multiple texture features yielded improved diagnostic accuracy than any single feature. The study implied potential value of myocardial texture features in differentiation of LVH etiologies by echocardiography. It may add some useful supplementary information to the routine TTE, and provide a cost-effective approach to aid diagnoses.
Acknowledgments
Funding: The work is supported by the National Natural Science Foundation of China (Grant No. 81871361, 61671281 and 61911530249), Shanghai East Hospital “leading talent project” (Grant No. DFRC2018024); Important Weak Subject Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZbr2017-09) and New Medicine Postgraduate Innovation Fund Program of Shanghai University.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4891
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4891
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4891). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Research Ethics Committee of Shanghai East Hospital (No. 2020-028) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swamy RS, Lang RM. Echocardiographic quantification of left ventricular mass: prognostic implications. Curr Cardiol Rep 2010;12:277-82. [Crossref] [PubMed]
- Cuspidi C, Facchetti R, Bombelli M, et al. Risk of mortality in relation to an updated classification of left ventricular geometric abnormalities in a general population: the Pamela study. J Hypertens 2015;33:2133-40. [Crossref] [PubMed]
- Weidemann F, Niemann M, Ertl G, et al. The different faces of echocardiographic left ventricular hypertrophy: clues to the etiology. J Am Soc Echocardiogr 2010;23:793-801. [Crossref] [PubMed]
- Angeli F, Reboldi G, Verdecchia P. Echocardiographic left ventricular hypertrophy: implications for clinicians. J Hypertens 2012;30:2279-84. [Crossref] [PubMed]
- Schofield R, Ganeshan B, Fontana M, et al. Texture analysis of cardiovascular magnetic resonance cine images differentiates aetiologies of left ventricular hypertrophy. Clin Radiol 2019;74:140-9. [Crossref] [PubMed]
- Hassani C, Saremi F, Varghese BA, et al. Myocardial Radiomics in Cardiac MRI. AJR Am J Roentgenol 2020;214:536-45. [Crossref] [PubMed]
- Mannil M, von Spiczak J, Manka R, et al. Texture Analysis and Machine Learning for Detecting Myocardial Infarction in Noncontrast Low-Dose Computed Tomography: Unveiling the Invisible. Invest Radiol 2018;53:338-43. [Crossref] [PubMed]
- Kagiyama N, Shrestha S, Cho JS, et al. A low-cost texture-based pipeline for predicting myocardial tissue remodeling and fibrosis using cardiac ultrasound. EBioMedicine 2020;54:102726. [Crossref] [PubMed]
- Kerut EK, Given M, Giles TD. Review of methods for texture analysis of myocardium from echocardiographic images: A means of tissue characterization. Echocardiography 2003;20:727-36. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Authors/Task Force members; Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Rodrigues JC, Amadu AM, Dastidar AG, et al. Prevalence and predictors of asymmetric hypertensive heart disease: insights from cardiac and aortic function with cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 2016;17:1405-13. [Crossref] [PubMed]
- Gaudron PD, Liu D, Scholz F, et al. The septal bulge--an early echocardiographic sign in hypertensive heart disease. J Am Soc Hypertens 2016;10:70-80. [Crossref] [PubMed]
- Arnold R, Schwendinger D, Jung S, et al. Left ventricular mass and systolic function in children with chronic kidney disease-comparing echocardiography with cardiac magnetic resonance imaging. Pediatr Nephrol 2016;31:255-65. [Crossref] [PubMed]
- Levin A. Anemia and left ventricular hypertrophy in chronic kidney disease populations: A review of the current state of knowledge. Kidney Int Suppl 2002.35-8. [Crossref] [PubMed]
- Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet 2016;388:276-84. [Crossref] [PubMed]
- Pinamonti B, Picano E, Ferdeghini EM, et al. Quantitative texture analysis in two-dimensional echocardiography: Application to the diagnosis of myocardial amyloidosis. J Am Coll Cardiol 1989;14:666-71. [Crossref] [PubMed]
- Skorton DJ, Collins SM, Woskoff SD, et al. Range and azimuth-dependent variability of image texture in two-dimensional echocardiograms. Circulation 1983;68:834-40. [Crossref] [PubMed]
- Smith SW, Wagner RF. Ultrasound speckle size and lesion signal to noise ratio: Verification of theory. Ultrason Imaging 1984;6:174-80. [Crossref] [PubMed]
- Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017;121:749-70. [Crossref] [PubMed]
- Ariga R, Tunnicliffe EM, Manohar SG, et al. Identification of Myocardial Disarray in Patients With Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. J Am Coll Cardiol 2019;73:2493-502. [Crossref] [PubMed]
- Izumaru K, Hata J, Nakano T, et al. Reduced Estimated GFR and Cardiac Remodeling: A Population-Based Autopsy Study. Am J Kidney Dis 2019;74:373-81. [Crossref] [PubMed]
- Moe SM. Calcium as a Cardiovascular Toxin in CKD-MBD. Bone 2017;100:94-99. [Crossref] [PubMed]