
SABR-COMET: a new paradigm of care lights up the twilight of metastatic disease
Not so long ago, the diagnosis of haematogenous metastases in a patient with a solid organ malignancy was synonymous with incurability and palliation. It was assumed that the appearance of the metastatic phenotype, even if at only one site, was the tip of the iceberg, and that it would only be a matter of time before synchronous but microscopic deposits would become clinically evident macroscopic metastases. As surmised by Hellman and Weichselbaum (1), on the balance of probabilities, in a population of patients with stage IV cancer, there could be a wide distribution of metastatic deposit numbers from one to many. If so, the ablative treatment of one or limited metastases (“oligometastases”) in addition to control of the primary might achieve long term disease control, or perhaps even cure. Further, if the metastasis was not solitary but in a life-limiting location such as the brain, ablation of the metastasis could at least prolong survival before the appearance of other competing metastatic risks for death. The first evidence in support of the oligometastatic concept was reported by Churchill and Barney in 1939, where they showed that a patient with synchronous metastatic kidney cancer was cured by nephrectomy and pulmonary metastasectomy (2). Later, randomized evidence in the setting of solitary brain metastasis demonstrated that surgical extirpation in addition to whole brain radiotherapy increased survival compared with radiotherapy alone (3). Ablation of brain oligometastases is a now well-established standard of care, and the concept might have gained widespread acceptance beyond the brain if it were not for potential morbidity of surgery in certain body sites (e.g., bone) or in the context of co-existing medical conditions (e.g., lung metastasectomy in a patient with limited cardiorespiratory reserve). The development of stereotactic ablative body radiotherapy (SABR), which is effective, non-invasive, relatively safe, convenient and widely applicable thus represented an attractive alternative to surgery in the oligometastatic setting. However high-level evidence to support its use to prolong survival has until now not been available.
The publication therefore of the SABR-COMET trial by Palma and colleagues is a welcome addition to the literature (4). Briefly, it is a randomized study that enrolled patients with a controlled primary tumour and one to five metastatic lesions. Patients were randomly assigned (1:2) to receive either palliative standard of care treatments alone (control group), or standard of care plus SABR to all metastatic lesions, with the primary endpoint of overall survival (OS). Thirty-three were assigned to the control group, and 66 in the treatment group. Median OS was 28 months in the control group versus 41 months in the SABR group (P=0.09). There was doubling of the progression free survival (PFS) in the SABR arm from 6 to 12 months (P=0.012). However, adverse events of grade 2 or worse occurred in three (9%) of 33 controls and 19 (29%) of 66 patients in the SABR group, an absolute increase of 20%, with treatment-related deaths occurring in three (4.5%) of 66 patients after SABR, compared with none in the control group. The authors emphasized that the study was designed as a Phase 2 screening trial (5). They employed a two-sided alpha of 0.20 in order to make a preliminary and non- definitive comparison between arms to determine if a Phase 3 trial was warranted.
With respect to number of metastases, most patients in the study (94%) had 1–3 metastatic deposits. Staging with FDG-PET was not mandatory, so the number of metastases may have been underestimated in some patients. At this time, it is not clear if there is a threshold number of metastases beyond which ablative treatment offers no additional benefit. A follow-on study, SABR-COMET 10 (NCT03721341) (6), is now recruiting for patients with 4–10 metastasis. The likelihood in a patient with a larger number of metastases, that all will be suitable for local ablative therapy does seem low, so accrual to this study will be watched with interest.
A perceived weakness of SABR-COMET is the larger number of prostate cancer cases in the SABR arm. The difference in OS might be explained by the favourable prognosis of this subset of patients, and the investigators addressed this with a sensitivity analysis, to explore the possibility that histology was a driver of the outcomes. In this analysis, after excluding all patients with prostate cancer, the hazard ratio for OS and PFS continued to favour the SABR arm. Another criticism is the three deaths in the SABR arm which were regarded as treatment related. On closer inspection, one death was from a subdural hematoma after repair of a gastric perforation in a patient with Crohn’s disease on steroid therapy. The second death was attributed to a large pulmonary abscess which developed in the treated location 1 year after SABR, with the patient declining active management of the abscess. The third patient succumbed after developing radiation pneumonitis 2 months after SABR. In the last case the chest had 2 lesions treated, one of which was centrally located. At a glance the first two treatment related deaths would not be typically expected secondary to SABR, whilst the last case appears to be convincingly attributable to SABR. It would be interesting to know if the patient had pre-existing underlying interstitial lung disease, a known risk factor for fatal radiation pneumonitis (7).
Two other Phase 2 randomized studies have addressed the question of local treatment for oligometastasis. Separate trials by Iyengar et al. (8) and Gomez et al. (9) evaluated the effect of local metastasis-directed therapies (either local radiotherapy, surgery or SABR) after first line systemic treatment in a more aggressive histology (non-small cell lung cancer). Both studies found significant improvements in progression-free survival (PFS). In Gomez’s initial report, the PFS advantage of local consolidative treatment over observation/maintenance therapy was so pronounced that the Data Safety and Monitoring Board recommended early trial closure after only 49 patients were recruited. Patients randomized to ablative therapy also had a lower incidence of additional metastases. In the prostate space, the STOMP RCT by Ost and colleagues (10) assigned 62 men with 3 or fewer metastatic lesions to metastasis directed treatment (MDT) versus surveillance. Androgen deprivation therapy (ADT) free survival was longer in the MDT group at 21 months compared to 13 months in the surveillance group. The ORIOLE trial (NCT02680587), recently presented at the 2019 American Society for Radiation Oncology Annual Meeting randomized 54 men with oligometastatic hormone-sensitive prostate cancer detected by conventional imaging to either SABR or observation (11). At 6 months, 61% had progressed in the observation arm vs. 19% in the SABR arm (P=0.005). A proportion in the SABR arm who had at least one-untreated lesion, those “subtotally consolidated”, had significantly worse PFS and had a higher risk of developing new metastasis. This suggests that total consolidation, or treating all macroscopic disease upfront can influence the development of microscopic disease. Overall, these randomized data suggests that local treatment of all disease improves cancer outcomes in oligometastatic patients.
In the setting of synchronous oligometastases, how aggressively should we treat the locoregional primary site? A Phase 3 clinical trial presented at the European Society for Medical Oncology 2019 Congress randomized 126 patients with metastatic nasopharyngeal carcinoma after initial chemotherapy to either radical loco-regional chemoradiation (to the primary and nodes to 66–70 Gy) or further chemotherapy (12). Early trial closure was recommended after a significant survival benefit was seen in the chemoradiation group at 40.2 months median OS versus chemotherapy alone at 24.5 months (HR 0.45, 95% CI: 0.25–0.80; P=0.007). The Phase 3 STAMPEDE randomized trial (13) in prostate cancer, the subset of patients with low volume metastatic disease had significantly improved survival through receipt of radiotherapy to the primary disease (HR 0.68, 95% CI: 0.52–0.90; P=0.007). With all these promising information, should we be treating the primary and all sites of oligometastasis? The next arm of the STAMPEDE trial, designed to investigate the role of metastasis directed therapy after standard of care, aims to answer this question (14). Our own local series of 49 patients with non-small cell lung cancer and synchronous solitary brain metastasis who were treated radically to both sites had a five year OS of 30%, which suggests that aggressive local therapy of all sites of disease can be associated with long-term survival and possible cure (15).
The findings of SABR-COMET await confirmation by studies currently underway (Table 1). However, since the findings of SABR-COMET are consistent with the seminal studies of Patchell et al. and Andrews et al. (23) in the context of brain oligometastases, it will be surprising if the ongoing Phase 3 trials do not show similar benefits. At the very least the study of Palma et al. provides proof of the principle that extracranial oligometastases are a real phenomenon for which we now have an effective, convenient and well tolerated treatment.
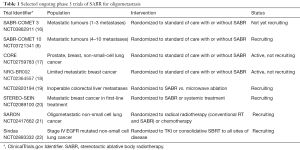
Full table
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Barney JD, Churchill EJ. Adenocarcinoma of the Kidney with Metastasis to the Lung: Cured by Nephrectomy and Lobectomy. Journal of Urology 1939;42:269-76. [Crossref]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 2005;23:7199-206. [Crossref] [PubMed]
- David P, Amsterdam University Medical Centre VS, British Columbia Cancer - Centre for the N, et al. Stereotactic Ablative Radiotherapy for Comprehensive Treatment of 4-10 Oligometastatic Tumors. 2019. Available online: .https://ClinicalTrials.gov/show/NCT03721341
- Chen H, Senan S, Nossent EJ, et al. Treatment-Related Toxicity in Patients With Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;98:622-31. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Phillips R, Lim SJ, Shi WY, et al. Primary Outcomes of a Phase II Randomized Trial of Observation Versus Stereotactic Ablative RadiatIon for OLigometastatic Prostate CancEr (ORIOLE). Int J Radiat Oncol Biol Phys 2019;105:681. [Crossref]
- Chen M, You R, You-Ping L, et al. 1108O Chemotherapy plus local-regional radiotherapy versus chemotherapy alone in primary metastatic nasopharyngeal carcinoma: A randomized, open-label, phase III trial. Ann Oncol 2019;30. [Crossref]
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018;392:2353-66. [Crossref] [PubMed]
- Choudhury A, Chen RC, Henry A, et al. STAMPEDE: Is Radiation Therapy to the Primary a New Standard of Care in Men with Metastatic Prostate Cancer? Int J Radiat Oncol Biol Phys 2019;104:33-5. [Crossref] [PubMed]
- Newman SJ, Bucknell N, Bressel M, et al. EP-1351 Long-term survival with FDG-PET directed therapy in NSCLC with synchronous solitary brain metastasis. Radiother Oncol 2019;133:S739. [Crossref]
- British Columbia Cancer A, London Regional Cancer Program C, Beatson Institute for Cancer Research S, et al. Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic (1-3 Metastases) Cancer. 2019. Available online: https://ClinicalTrials.gov/show/NCT03862911
- Royal Marsden NHSFT, Institute of Cancer Research UK, National Health Service UK. Conventional Care Versus Radioablation (Stereotactic Body Radiotherapy) for Extracranial Oligometastases. 2016. Available online: https://ClinicalTrials.gov/show/NCT02759783
- Oncology NRG, National Cancer I. Standard of Care Therapy With or Without Stereotactic Radiosurgery and/or Surgery in Treating Patients With Limited Metastatic Breast Cancer. 2015. Available online: https://ClinicalTrials.gov/show/NCT02364557
- Istituto Clinico H. A Trial on SBRT Versus MWA for Inoperable Colorectal Liver Metastases (CLM). 2016. Available online: https://ClinicalTrials.gov/show/NCT02820194
- Gustave Roussy CCGP. Trial of Superiority of Stereotactic Body Radiation Therapy in Patients With Breast Cancer. 2014. Available online: https://ClinicalTrials.gov/show/NCT02089100
- University College L, Cancer Research UK. Stereotactic Ablative Radiotherapy for Oligometastatic Non-small Cell Lung Cancer. 2015. Available online: https://ClinicalTrials.gov/show/NCT02417662
- Sichuan Provincial People's H. Stereotactic Body Radiation Therapy (SBRT) in Newly Diagnosed Advanced Staged Lung Adenocarcinoma (Sindas). 2016. Available online: https://ClinicalTrials.gov/show/NCT02893332
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]


