
Long-term outcomes of childhood onset Noonan compared to sarcomere hypertrophic cardiomyopathy
Introduction
Whereas adult hypertrophic cardiomyopathy (HCM) is mainly caused by mutations in genes encoding for components of the sarcomere, neonatal and childhood onset HCM is often associated with Noonan syndrome. Within the past decade, extensive clinical and experimental research has contributed to our understanding of sarcomere-associated HCM (S-HCM). European (1,2) and North-American (1,3) guidelines facilitate the management of patients with S-HCM, however those exclude patients with syndromic HCM and far less is known about outcome, clinical course and cardiac pathology of patients with Noonan syndrome-associated hypertrophic cardiomyopathy (N-HCM). A detailed understanding of disease course is necessary to provide optimal counseling, risk stratification and treatment.
Noonan syndrome has a prevalence of 1:1,000–1:2,500 (4,5) and belongs to the spectrum of diseases called “RASopathies” (alternatively called neuro-cardio-facial-cutaneous syndromes). It is caused by autosomal-dominant mutations in genes of the mitogen-activated protein kinase (MAPK) signaling pathway (5). Patients present with characteristic patterns of facial anomalies, short stature, congenital heart defects, hematologic disorders, and mental retardation (6).
HCM occurs in about 15% (7) to 20% (8,9) of patients with Noonan-syndrome. Mutations in the PTPN11, RAF1 and RIT1 genes are most often associated with the occurrence of HCM in Noonan-patients (9-12). Patients present clinically with myocardial hypertrophy (13), right and/or left ventricular outflow tract (LVOT) obstruction, diastolic and/or systolic dysfunction (7), and rarely with arrhythmia (8,9). Similar to S-HCM (14), fibrotic myocardial changes (13,15) and myocyte disarray (13,16) have been described in N-HCM. Therapeutic options are limited to medical treatment of heart failure symptoms, or surgical septal myectomy to relieve drug-refractory severe left and/or right ventricular outflow tract obstruction (17,18). New therapies directly targeting the RAS/MAPK pathways are currently under investigation (19-21).
This single center cohort study compares long-term outcome and cardiac pathology between patients with genetically diagnosed childhood onset N-HCM and S-HCM.
Methods
Study design
All patients with childhood HCM presenting at the German Heart Center Munich, a tertiary care university hospital, between January 1978 and July 2018 were included in the study.
Demographic, clinical, molecular genetic testing result, transthoracic echocardiographic (TTE), electrocardiographic (ECG), 24-hour ambulatory Holter ECG, cardiopulmonary exercise testing (CPET), cardiovascular magnetic resonance (CMR), surgical, histopathologic and other patient-related data were collected retrospectively by review of patients’ charts within 1, 5 and 10 years after diagnosis, and at last follow-up.
Molecular genetic DNA-based analysis was carried out as recommended by European and North-American guidelines (2,22-24) in accredited laboratories only and criteria for assessing variant pathogenicity were based on the variant type, variant database, literature review, frequency in the general population, and in silico analysis according to the ACMG (American College of Medical Genetics and Genomics) guidelines (25,26).
Patients were classified into two groups for comparison: patients with a genetic or clinical diagnosis of Noonan syndrome and HCM (N-HCM) (4) and patients with a genetic diagnosis of sarcomere protein-associated HCM (S-HCM) (2,27).
Primary endpoint was death. Secondary endpoints were survival until hospitalization for heart failure, survival until hospitalization for intervention, and survival until first severe arrhythmic event. Intervention was defined as either percutaneous cardiac intervention or cardiac operation. Severe arrhythmic event was defined as sudden cardiac death, aborted sudden cardiac death, appropriate ICD discharge or sustained ventricular or supraventricular tachycardia.
Inclusion and exclusion criteria
All children with a primary diagnosis of HCM between birth and the age of 18 years and a positive genetic result for HCM or Noonan syndrome, or a clinical diagnosis of Noonan syndrome were included in the study (also see Supplementary files for further details). Patients with other complex structural heart disease and with other genetic, metabolic or neuromuscular disorders were excluded.
Clinical data, imaging and histopathology
Data were collected as previously described (28). Please see Supplementary files for detailed description of imaging, ECG, CPET, and histopathology.
Statistics
Statistical analysis was performed with the SPSS software version 22.0.0 (SPSS Inc., IBM Company, Chicago, Illinois, USA). The time-related probability of survival until primary endpoint and secondary endpoints were estimated with the Kaplan-Meier method and compared by the log-rank test between groups. Data were censored at the time of event or the latest time of follow-up. Continuous variables are expressed as means (95% confidence interval) or median (range: minimum–maximum), according to sample distribution. Differences between N-HCM and S-HCM patients were analyzed by independent t-test for normally distributed or Mann-Whitney Wilcoxon test for not normally distributed data. Longitudinal data were compared with paired t-test or Wilcoxon signed rank tests where appropriate. Categorical variables are given as percentages of group totals and were analyzed by Pearson Chi Square or Fisher exact test. A P value of <0.05 (two-sided) was considered statistically significant.
Ethics
This study was approved by the Technical University of Munich Institutional Review Board (ethical approval number 243/17S, 10/16/2017).
Results
Patients characteristics
Medical records were screened for of all patients diagnosed with childhood onset HCM and 75 patients were identified. Of those, a genetic or clinical diagnosis of Noonan syndrome (N=24) or Noonan syndrome with multiple lentigines (N=5) was made in 29 patients and a genetic diagnosis of sarcomere-protein associated HCM in 34 patients (Figure 1). Twelve patients were excluded from further analysis due to lacking clear clinical characterization or genetic information. On last follow-up, TTE and ECG were performed in all patients, genetic testing, 24-hour Holter ECG, CPET, CMR, and histopathology in a subset of patients. A pathogenic mutation was identified in 55 out of 63 patients tested (87.3%). Pathogenic variants in the genes PTPN11 (about half of patients presenting with Noonan syndrome and multiple lentigines, NS-ML, or previously called LEOPARD syndrome) and RAF1 were the most prominent mutations detected in N-HCM. S-HCM patients were most commonly affected with mutations in MYH7 and MYBPC3. Four S-HCM patients carried more than one mutation (Figure 1).
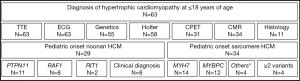
Gender distribution was similar between N-HCM and S-HCM patients. Most N-HCM patients were diagnosed within their first year of life at a younger age as compared to S-HCM patients, who were mostly first diagnosed within early school years. Clinical follow-up time after diagnosis was similar between groups and was more than 10 years in half of all patients.
Family history was negative in most N-HCM patients and there was no family history of sudden cardiac death in this group.
N-HCM patients were more likely to carry a concomitant cardiac diagnosis, which included right ventricular outflow tract obstruction, mitral valve abnormalities and pulmonic valve pathology.
No S-HCM but most N-HCM patients presented with additional clinical features, such as facial dysmorphologies, short stature, or skin lesions (Table 1).

Full table
Clinical outcome
Primary study endpoint was death. There were two deaths in the N-HCM (6.9%) and no death in the S-HCM group (Figure 2A). One NS-ML patient carrying a PTPN11 mutation died in the immediate postoperative period after septal myectomy from multi-organ failure at the age of 4.3 months. The second patient carried a clinical diagnosis of Noonan syndrome and died at 11.3 years of age due to heart failure secondary to undiagnosed supraventricular tachycardia in an outside hospital.
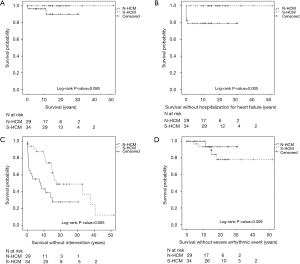
Survival without hospitalization for heart failure or intervention was shorter in N-HCM as compared to S-HCM patients (Figure 2B,C). N-HCM patients were more likely to require septal myectomy compared to S-HCM patients (Table 1). Concomitant procedures included valve surgery in 10 N-HCM and 1 S-HCM patient, right ventricular outflow tract resection in 2 N-HCM patients, and relief of myocardial muscle bridges in 1 S-HCM patient.
The occurrence of arrhythmias on ECG, 24-hour ECG or CPET, including supraventricular and ventricular extra beats and tachycardia, was equal between groups (Table 1). One of 29 (3.4%) N-HCM and 5 of 34 (14.7%) S-HCM patients reached the secondary endpoint severe arrhythmic event (P=0.205, Fishers exact test, and Figure 2D). Only S-HCM patients received an ICD for primary prevention in most of those patients and half of the patients with ICD experienced at least one appropriate discharge (Table 1).
Clinical features and cardiac pathology
Compared to S-HCM patients, N-HCM patients presented in a higher NYHA/Ross functional class at the time of diagnosis which improved over time (Figure 3A). Peak VO2 on CPET (Table 1) and the number of cardiac medications on last follow-up were similar between groups. 56% of N-HCM and 71% of S-HCM patients used beta-blockers on last follow-up (P=0.5, Fishers exact test).
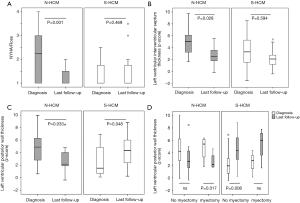
Relative thickness of the left ventricular posterior wall and interventricular septum decreased over time in N-HCM and increased in S-HCM patients (Figure 3B,C), even if separated into groups of patients without versus after surgical septal myectomy (Figure 3D).
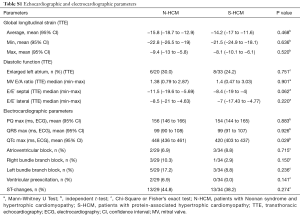
There were no differences in systolic or diastolic ventricular function between groups, as assessed by ventricular ejection fraction, global longitudinal strain, left atrial size, mitral valve E/A ratio, or septal and lateral E/E’ on TTE, respectively (Table S1). Evidence of focal or interstitial fibrosis as assessed by histopathology or late gadolinium enhancement (LGE) and T1 map on CMR, respectively, was found in both groups (Table 1). Apical aneurysm was detected on CMR in 3 N-HCM and 1 S-HCM patient (Table 1).
Full table
On sub analysis, there were no differences in patient characteristics, clinical features and cardiac pathology between N-HCM patients carrying distinct mutations (data not shown).
Discussion
General outcome and natural history of Noonan patients has been described before (7-9,18,29-32), but most of those data lack genetic information and detailed clinical description, such as time course of disease development, clinical functional status, imaging, electrophysiologic information, and a direct comparison to patients with childhood onset sarcomere-protein related HCM.
This is the first study to directly compare outcome and detailed cardiac features between patients with childhood onset HCM carrying a genetic diagnosis of Noonan syndrome and patients with sarcomere protein-associated HCM in a large single center cohort.
The main findings of the present study are a low mortality and a favorable long-term outcome of N-HCM patients despite presentation during infancy and despite significant morbidity during the first years of life. The current data also provide evidence that although there are similar features on CMR imaging and histopathology between N-HCM and S-HCM patients, there is, in contrast to S-HCM patients, no progression but rather regression of myocardial hypertrophy. There was a trend to a lower occurrence of severe arrhythmic events in N-HCM as compared to S-HCM patients.
The low mortality of below 7% described in this study differs from most previous study reports (8,32-35), which state higher mortality rates specifically in patients diagnosed during early infancy and presenting with severe heart failure. The main reason for the discrepancy of findings is that data from the current study derive from a tertiary care referral center. Very sick infants who deceased prior at an outside hospital or children with infant HCM of other etiologies are not reflected in the present statistics. Second, aggressive medical and interventional treatments during early disease course as depicted by the secondary endpoint Kaplan-Meier-curves in the current study might have contributed to low mortality in N-HCM patients.
Compared to S-HCM patients, N-HCM patients present with worse clinical status and at an earlier age, requiring hospital admissions and interventions early in life. The reason for intervention included relief of LVOT obstruction in most of N-HCM patients, and valve operation and/or relief of right ventricular outflow tract obstruction in a subset of those patients. In comparison, cardiac procedures other than relief of LVOT obstruction were rare in S-HCM patients.
Clinical functional status improved in N-HCM patients over time and in contrast to S-HCM patients there is no progression of left ventricular hypertrophy standardized to body surface area (z-scores) assessed by TTE over time beyond childhood in most of N-HCM patients. Instead, both left ventricular posterior wall and interventricular septal z-scores decreased over time in N-HCM patients. Because surgical septal myectomy was performed in most of those patients, comparison of myocardial z-scores between diagnosis and last follow-up was also done separately for patients requiring surgical septal myectomy and those who did not. This sub analysis again showed that myocardial z-scores regressed in both groups, but due to small patient numbers this did not reach statistical significance in patients not undergoing septal myectomy. Spontaneous regression of HCM was also reported by others (9), but detailed TTE findings were not presented in that study. In contrast, non-syndromic familial HCM due to sarcomere protein-associated mutations tend to worsen over time (36). Distinct underlying molecular pathology might account for this clinical observation. Altered biophysical properties and activation of pro-hypertrophic and -fibrotic transcription factors are known to cause HCM in patients with sarcomere protein-associated mutations (37,38). Alteration in the RAS/MAPK signaling pathway cause hypertrophy by a distinct mechanism, which might be more pronounced during perinatal development and less in post-natal life (39,40).
Histopathologic results of the current study showed that there was a similar amount of quantified fibrosis on histopathology performed on myocardial specimens. Similar histopathologic changes were described in small case series (30) or single patient case reports (13,16). The fact that specimens in the current study were gained at the time of septal myectomy during early childhood in most of the N-HCM patients provides evidence that in contrast to S-HCM, in which pathologic myocardial remodeling occurs over time, fibrotic changes in N-HCM patients are already present during early disease course in severely hypertrophic areas that require surgical resection. Myocardial changes persist in N-HCM patients as CMR on follow-up shows LGE as a correlate for focal fibrosis and increased interstitial fibrosis assessed by CMR T1 mapping.
CMR also showed the presence of apical aneurysms in three N-HCM patients. With the exception of one case report (15), there are no studies on CMR findings in N-HCM patients so that the overall prevalence is unknown in this population. Apical aneurysms are associated with increased morbidity in patients with sarcomere-related HCM (41,42) but the significance in N-HCM patients has not been described yet. In the current study, none of the three N-HCM patients with apical aneurysms required specific treatment or experienced severe arrhythmic or thromboembolic events at this point.
HCM is the main reason for sudden arrhythmic death in adolescents and young adults (43,44). No N-HCM patient suffered sudden cardiac death, aborted sudden death, or required an ICD in the current study. However, severe arrhythmias, including sustained supraventricular tachycardia and non-sustained ventricular tachycardia occurred in 3 N-HCM patients. In contrast, 2 patients with S-HCM presented after cardiopulmonary resuscitation and half of S-HCM patients with an ICD had appropriate discharges. Those findings, together with the findings reported by other authors (8,9,16) suggest that N-HCM patients are at lower risk for sudden cardiac death. Ventricular tachycardia and sudden death were reported in another N-HCM population (7), but those patients had additional major cardiac anomalies which could explain their increased risk for malignant arrhythmias. Risk stratification for sudden death is of highest importance in patients with HCM (45-48), but data about risk stratification in N-HCM patients are lacking and numbers of N-HCM patients experiencing severe arrhythmic events in the current study were too small to evaluate for risk factors in this population. Therefore, risk stratification for malignant arrhythmias at this point needs to be performed on an individual base.
Taken together, clinical outcome of affected patients is directly related to distinct disease courses of N-HCM patients compared to S-HCM patients. N-HCM patients can be severely affected in the perinatal period and during infancy which requires aggressive medical or interventional/surgical management at this early stage in life. This contrasts with S-HCM patients in whom surgical interventions are less frequently required during early childhood but in whom sudden cardiac death risk stratification and possibly implantation of an ICD becomes critical during adolescence and young adulthood.
The current study’s observations might impact clinical management of N-HCM patients. In contrast to S-HCM patients, treatment of LVOT obstruction or heart failure in N-HCM patients mostly occurs early in life during infancy. Disturbed RAS signaling causes HCM in Noonan patients (40,49) and small-molecule therapies inhibiting the appropriate pathway may have the potential to prevent or reverse cardiac pathology in affected patients. Inhibition of protein kinase B (Akt) (19) or mTOR (40,50) has shown to be effective treatment options in RASopathy mouse models and one case report describes improvement of heart failure symptoms in a critically ill infant with Noonan syndrome and multiple lentigines by treatment with the mTOR inhibitor rapamycin (21). However, those personalized therapies are still experimental and reserved for patients with critical disease.
Study limitations
The usual limitations of a single center retrospective study, such as selection bias and inter-observer variability for clinical and echocardiographic evaluations performed by different physicians, apply to this data analysis. The prevalence of childhood HCM in general and in the setting of Noonan syndrome low. The small number of subjects limits statistical power and the feasibility of subgroup analysis in the cohort of the current study. Some associations were clinically but not statistically significant and do only have descriptive character. Due to the strict inclusion criteria of a genetic diagnosis in the S-HCM group, children with familial non-syndromic HCM and a negative genetic result were not included in this analysis and data might thus be not representative for this group. CMR and CPET were not performed in all patients due to age or body size, or other contraindications. CMR findings were used in this study to assess the amount and distribution of focal and interstitial fibrosis. Despite good correlation of imaging and histopathologic changes (51), CMR might not reflect true cellular myocardial changes.
Conclusions
N-HCM patients have a favorable long-term outcome with overall low mortality despite significant morbidity requiring hospitalization and intervention early in life. In contrast to S-HCM patients there is stagnation of myocardial hypertrophy and a low risk for malignant arrhythmias over time in the N-HCM population. Findings of this study impact counseling of patients with Noonan syndrome and childhood onset HCM.
Supplementary
Online supplement patients and methods
Inclusion and exclusion criteria
All children with a primary diagnosis of hypertrophic cardiomyopathy (HCM) between birth and the age of 18 years were included in the study. The diagnosis of HCM was based on clinical evaluation and cardiac catheterization or transthoracic echocardiography (TTE) detecting myocardial hypertrophy (defined as a z-score of greater than 2) in the absence of another cardiac or systemic disease causing the degree of left ventricular hypertrophy identified (2,52).
Since Noonan syndrome can be diagnosed by typical syndromic features, such as craniofacial abnormalities, short stature, failure to thrive, etc., all patients with a clinical or molecular genetic diagnosis of Noonan syndrome or Noonan syndrome with multiples lentigines (NS-ML) were included in the Noonan syndrome HCM cohort. In the group of sarcomere protein-associated HCM, only patients with molecular testing positive for a pathogenic or likely pathogenic variant were included, because of the lack of clear clinical features in this cohort and because HCM in genotype-negative patients could have distinct etiologies, such as mitochondriopathy, Fabry disease, or storage disease.
Clinical data, imaging and histopathology
The total number of clinical patients’ visits varied according to patients ‘compliance and the physicians’ practice. Data were collected retrospectively from regular clinical visits and data were recorded from the last possible timepoint within 1, 5, and 10 years after diagnosis, and at last follow-up. Hence, study data from up to 4 studies were recorded for analysis.
TTE was performed using standard equipment in routine clinical practice and using standard views according to the American Society of Echocardiography guidelines (53-55). Echopac Software (General Electric, Vingmed, Horten, Norway) was used for offline-analysis. Measurements of left ventricular wall thickness were standardized to body surface area (z-scores), which describe how many standard deviations lie above or below a size-matched population given a specific measurement. Given the large variability of age and size in children, z-scores are commonly used in pediatric cardiology. The left ventricular outflow tract (LVOT) gradient was assessed under resting conditions, using continuous wave Doppler echocardiography (56,57).
On 12-lead ECG, heart rate, arrhythmia, time intervals, patterns of myocardial ischemia and hypertrophy were documented (58). Occurrence of arrhythmia was assessed additionally by ambulatory Holter ECG, if existing, from the memory of a permanent pacemaker, recording activity and shock frequency.
Cardiopulmonary exercise testing (CPET) was performed in sitting position on a bike-ergometer. A standardized testing protocol was performed and physical working capacity, peak oxygen uptake (peak VO2), evidence of myocardial ischemia or arrhythmia and escalation of heart rate or blood pressure during or after exertion were recorded (59).
CMR was performed on a 1.5 Tesla MR scanner (Magnetom Avanto, Siemens Healthcare, Software Version VD13, Erlangen, Germany). Cine images (balanced steady-state free precession) were acquired in short axis and four chamber orientations, in breath hold or in free breathing in younger children not able to hold the breath, to evaluate the ventricular volume, ventricular mass, ejection fraction and regional wall anomalies. Late Gadolinium Enhancement (LGE) was acquired using a T1-weighted phase-sensitive inversion recovery sequence 10 minutes after intravenous administration of an extracellular MR contrast agent (Gadopentetat) to detect focal fibrosis (60).
Native and post contrast T1 mapping, using a modified look-locker inversion recovery sequence (MOLLI) (61), with non-rigid motion correction reconstruction, were assessed in short axis and four chamber orientation. Image quality was assessed by revising T1 maps and error maps of the region of interest. Extracellular volume (ECV) was calculated as previously described (62).
Criteria for surgical intervention at the German Heart Center in Munich were LVOT obstruction ≥50 mm Hg at rest or with provocative maneuvers, associated with New York Heart Association (NYHA) functional classes/Ross ≥ III despite maximum medical management (1,63). The septal myectomy in the present cohort was performed as previously described (28,64,65).
Cardioverter-defibrillator (ICD) was implanted for secondary prevention in HCM patients who have survived a cardiac arrest caused by ventricular fibrillation or sustained ventricular tachycardia according to the European (2) and North-American guidelines (3). ICD implantation as primary prevention included individual risk assessment based on conventional risk factors, such as massive LV hypertrophy, syncope of unknown etiology, family history of sudden death <40 years/age, non-sustained ventricular tachycardia, and abnormal blood pressure response on stress test (66-69). Additionally, the online available risk prediction model for sudden cardiac death in HCM (http://www.doc2do.com/hcm/webHCM.html) was used for patients older than 16 years of age (70).
Histopathologic examination of myectomy specimens was performed in a blinded fashion by a cardiovascular pathologist. Presence of myocyte disarray was assessed on hematoxylin/eosin stained slides. For the quantification of cardiac fibrosis, paraffin-embedded specimens were stained with Masson’s trichrome, reflecting myocyte necrosis as well as interstitial fibrosis, and were analyzed by light microscopy using the interactive program Quantuepatho. Fibrotic areas were digitally dissected on 5 µm thick sections. The percentage area of fibrosis in the section was evaluated by dividing the sum of the fibrotic areas of the section by that of the total tissue area as described previously (71).
Acknowledgments
We would like to thank and dedicate this work to PD Dr. S Fratz who passed away after initiating this study. All phases of this study were supported by the Gerd-Killian Award Deutsche Herzstiftung e.V. (CM Wolf).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Technical University of Munich Institutional Review Board (ethical approval number 243/17S, 10/16/2017).
References
- Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003;24:1965-91. [Crossref] [PubMed]
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;58:2703-38. [Crossref] [PubMed]
- Romano AA, Allanson JE, Dahlgren J, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics 2010;126:746-59. [Crossref] [PubMed]
- Tartaglia M, Gelb BD. Noonan syndrome and related disorders: genetics and pathogenesis. Annu Rev Genomics Hum Genet 2005;6:45-68. [Crossref] [PubMed]
- Gelb BD, Tartaglia M. Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet 2006;15:R220-6. [Crossref] [PubMed]
- Colquitt JL, Noonan JA. Cardiac findings in Noonan syndrome on long-term follow-up. Congenit Heart Dis 2014;9:144-50. [Crossref] [PubMed]
- Shaw AC, Kalidas K, Crosby AH, et al. The natural history of Noonan syndrome: a long-term follow-up study. Arch Dis Child 2007;92:128-32. [Crossref] [PubMed]
- Prendiville TW, Gauvreau K, Tworog-Dube E, et al. Cardiovascular disease in Noonan syndrome. Arch Dis Child 2014;99:629-34. [Crossref] [PubMed]
- Tartaglia M, Kalidas K, Shaw A, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet 2002;70:1555-63. [Crossref] [PubMed]
- Pandit B, Sarkozy A, Pennacchio LA, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet 2007;39:1007-12. [Crossref] [PubMed]
- Aoki Y, Niihori T, Banjo T, et al. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet 2013;93:173-80. [Crossref] [PubMed]
- Fahrner JA, Frazier A, Bachir S, et al. A rasopathy phenotype with severe congenital hypertrophic obstructive cardiomyopathy associated with a PTPN11 mutation and a novel variant in SOS1. Am J Med Genet A 2012;158A:1414-21. [Crossref] [PubMed]
- Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 2001;104:557-67. [Crossref] [PubMed]
- Hudsmith LE, Petersen SE, Francis JM, et al. Hypertrophic cardiomyopathy in Noonan Syndrome closely mimics familial hypertrophic cardiomyopathy due to sarcomeric mutations. Int J Cardiovasc Imaging 2006;22:493-5. [Crossref] [PubMed]
- Burch M, Mann JM, Sharland M, et al. Myocardial disarray in Noonan syndrome. Br Heart J 1992;68:586-8. [Crossref] [PubMed]
- Noonan JA. Noonan syndrome and related disorders: alterations in growth and puberty. Rev Endocr Metab Disord 2006;7:251-5. [Crossref] [PubMed]
- Nugent AW, Daubeney PE, Chondros P, et al. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation 2005;112:1332-8. [Crossref] [PubMed]
- Wang J, Chandrasekhar V, Abbadessa G, et al. In vivo efficacy of the AKT inhibitor ARQ 092 in Noonan Syndrome with multiple lentigines-associated hypertrophic cardiomyopathy. PLoS One 2017;12:e0178905. [Crossref] [PubMed]
- Duat-Rodriguez A, Hernandez-Martin A. Update on the treatment of RASopathies. Rev Neurol 2017;64:S13-7. [PubMed]
- Hahn A, Lauriol J, Thul J, et al. Rapidly progressive hypertrophic cardiomyopathy in an infant with Noonan syndrome with multiple lentigines: palliative treatment with a rapamycin analog. Am J Med Genet A 2015;167A:744-51. [Crossref] [PubMed]
- Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011;8:1308-39. [Crossref] [PubMed]
- Charron P, Arad M, Arbustini E, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2010;31:2715-26. [Crossref] [PubMed]
- Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017;121:749-70. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Amendola LM, Jarvik GP, Leo MC, et al. Performance of ACMG-AMP Variant-Interpretation Guidelines among Nine Laboratories in the Clinical Sequencing Exploratory Research Consortium. Am J Hum Genet 2016;99:247. [Crossref] [PubMed]
- Olivotto I, d'Amati G, Basso C, et al. Defining phenotypes and disease progression in sarcomeric cardiomyopathies: contemporary role of clinical investigations. Cardiovasc Res 2015;105:409-23. [Crossref] [PubMed]
- Schleihauf J, Cleuziou J, Pabst von Ohain J, et al. Clinical long-term outcome of septal myectomy for obstructive hypertrophic cardiomyopathy in infants. Eur J Cardiothorac Surg 2018;53:538-44. [Crossref] [PubMed]
- Wilkinson JD, Lowe AM, Salbert BA, et al. Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: a study from the Pediatric Cardiomyopathy Registry. Am Heart J 2012;164:442-8. [Crossref] [PubMed]
- Poterucha JT, Johnson JN, O'Leary PW, et al. Surgical Ventricular Septal Myectomy for Patients With Noonan Syndrome and Symptomatic Left Ventricular Outflow Tract Obstruction. Am J Cardiol 2015;116:1116-21. [Crossref] [PubMed]
- Calcagni G, Adorisio R, Martinelli S, et al. Clinical Presentation and Natural History of Hypertrophic Cardiomyopathy in RASopathies. Heart Fail Clin 2018;14:225-35. [Crossref] [PubMed]
- Alexander PMA, Nugent AW, Daubeney PEF, et al. Long-Term Outcomes of Hypertrophic Cardiomyopathy Diagnosed During Childhood: Results From a National Population-Based Study. Circulation 2018;138:29-36. [Crossref] [PubMed]
- Calcagni G, Limongelli G, D'Ambrosio A, et al. Cardiac defects, morbidity and mortality in patients affected by RASopathies. CARNET study results. Int J Cardiol 2017;245:92-8. [Crossref] [PubMed]
- Skinner JR, Manzoor A, Hayes AM, et al. A regional study of presentation and outcome of hypertrophic cardiomyopathy in infants. Heart 1997;77:229-33. [Crossref] [PubMed]
- Suda K, Kohl T, Kovalchin JP, et al. Echocardiographic predictors of poor outcome in infants with hypertrophic cardiomyopathy. Am J Cardiol 1997;80:595-600. [Crossref] [PubMed]
- Maron BJ. Hypertrophic cardiomyopathy. Lancet 1997;350:127-33. [Crossref] [PubMed]
- Fatkin D, McConnell BK, Mudd JO, et al. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin Invest 2000;106:1351-9. [Crossref] [PubMed]
- Teekakirikul P, Eminaga S, Toka O, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest 2010;120:3520-9. [Crossref] [PubMed]
- Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet 2001;29:465-8. [Crossref] [PubMed]
- Schramm C, Fine DM, Edwards MA, et al. The PTPN11 loss-of-function mutation Q510E-Shp2 causes hypertrophic cardiomyopathy by dysregulating mTOR signaling. Am J Physiol Heart Circ Physiol 2012;302:H231-43. [Crossref] [PubMed]
- Maron MS, Finley JJ, Bos JM, et al. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 2008;118:1541-9. [Crossref] [PubMed]
- Xiao Y, Wang LP, Yang YK, et al. Clinical Profile and Prognosis of Left Ventricular Apical Aneurysm in Hypertrophic Cardiomyopathy. Am J Med Sci 2016;351:101-10. [Crossref] [PubMed]
- Maron BJ, Haas TS, Ahluwalia A, et al. Demographics and Epidemiology of Sudden Deaths in Young Competitive Athletes: From the United States National Registry. Am J Med 2016;129:1170-7. [Crossref] [PubMed]
- Ostman-Smith I, Wettrell G, Keeton B, et al. Age- and gender-specific mortality rates in childhood hypertrophic cardiomyopathy. Eur Heart J 2008;29:1160-7. [Crossref] [PubMed]
- Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000;36:2212-8. [Crossref] [PubMed]
- Monserrat L, Elliott PM, Gimeno JR, et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003;42:873-9. [Crossref] [PubMed]
- Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342:1778-85. [Crossref] [PubMed]
- Elliott PM, Gimeno Blanes JR, Mahon NG, et al. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 2001;357:420-4. [Crossref] [PubMed]
- Gelb BD, Tartaglia M. RAS signaling pathway mutations and hypertrophic cardiomyopathy: getting into and out of the thick of it. J Clin Invest 2011;121:844-7. [Crossref] [PubMed]
- Marin TM, Keith K, Davies B, et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest 2011;121:1026-43. [Crossref] [PubMed]
- Fontana M, White SK, Banypersad SM, et al. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson 2012;14:88. [Crossref] [PubMed]
- Rakowski H, Sasson Z, Wigle ED. Echocardiographic and Doppler assessment of hypertrophic cardiomyopathy. J Am Soc Echocardiogr 1988;1:31-47. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Weidemann F, Eyskens B, Jamal F, et al. Quantification of regional left and right ventricular radial and longitudinal function in healthy children using ultrasound-based strain rate and strain imaging. J Am Soc Echocardiogr 2002;15:20-8. [Crossref] [PubMed]
- Kühn A, De Pasquale Meyer G, Müller J, et al. Tricuspid valve surgery improves cardiac output and exercise performance in patients with Ebstein's anomaly. Int J Cardiol 2013;166:494-8. [Crossref] [PubMed]
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295-303. [Crossref] [PubMed]
- Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol 1995;26:1699-708. [Crossref] [PubMed]
- Rijnbeek PR, Witsenburg M, Schrama E, et al. New normal limits for the paediatric electrocardiogram. Eur Heart J 2001;22:702-11. [Crossref] [PubMed]
- American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211-77. [Crossref] [PubMed]
- Kellman P, Arai AE, McVeigh ER, et al. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med 2002;47:372-83. [Crossref] [PubMed]
- Messroghli DR, Radjenovic A, Kozerke S, et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141-6. [Crossref] [PubMed]
- Kellman P, Wilson JR, Xue H, et al. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 2012;14:63. [Crossref] [PubMed]
- Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004;350:1320-7. [Crossref] [PubMed]
- Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy. Ann Surg 1961;154:181-9. [Crossref] [PubMed]
- Maron BJ, Nishimura RA, Danielson GK. Pitfalls in clinical recognition and a novel operative approach for hypertrophic cardiomyopathy with severe outflow obstruction due to anomalous papillary muscle. Circulation 1998;98:2505-8. [Crossref] [PubMed]
- O'Mahony C, Tome-Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart 2013;99:534-41. [Crossref] [PubMed]
- Di Salvo G, Pacileo G, Limongelli G, et al. Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr 2010;23:581-90. [Crossref] [PubMed]
- O'Mahony C, Lambiase PD, Rahman SM, et al. The relation of ventricular arrhythmia electrophysiological characteristics to cardiac phenotype and circadian patterns in hypertrophic cardiomyopathy. Europace 2012;14:724-33. [Crossref] [PubMed]
- Biagini E, Lorenzini M, Olivotto I, et al. Effects of myocardial fibrosis assessed by MRI on dynamic left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy: a retrospective database analysis. BMJ Open 2012;2. [Crossref] [PubMed]
- O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010-20. [Crossref] [PubMed]
- Mueller KA, Mueller II, Eppler D, et al. Clinical and histopathological features of patients with systemic sclerosis undergoing endomyocardial biopsy. PLoS One 2015;10:e0126707. [Crossref] [PubMed]


