Impact of comorbidity burden on morbidity following thoracoscopic lobectomy: a propensity-matched analysis
Introduction
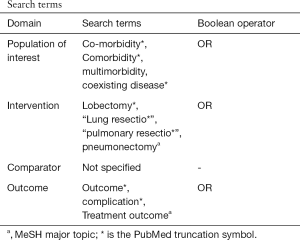
As the population ages, the proportion of lung cancer patients with multiple comorbidities is rising (1). While some major comorbid diseases, such as cardiopulmonary and renal diseases, pose higher risks of mortality and morbidity following major lung resection, the impact of overall comorbidity burden on postoperative outcomes has not been thoroughly examined (2-4). So far, Charlson comorbidity index (CCI) is the most widely used method of assessing a patient’s overall comorbidity burden in clinical research (5). Systematic reviews of published data demonstrated that controversy still exists regarding the relationship of higher comorbidity burden measured by CCI with nonfatal complications after major lung resection, though unequivocal data indicate it as a predictor of postoperative mortality (see the protocol of literature review in the supplementary Appendix 1) (6-10).
Thoracoscopic lobectomy has been introduced in the early 1990s and increasingly adopted since 2005 (11). Currently, it is the preferred surgical approach for treatment of early stage non-small cell lung cancer (NSCLC). Owing to less trauma to the chest wall and better preservation of lung function in the initial postoperative period, thoracoscopic lobectomy is associated with reduced postoperative mortality and morbidity, particularly in high-risk patients (12,13). In patients with an increased comorbidity burden, thoracoscopic lobectomy has been found feasible and safe in some early case series (14). Given the positive effect of a thoracoscopic approach on improving postoperative outcomes, the question has arisen as to whether the magnitude and impact of an increased comorbidity burden may be different comparing open and minimally invasive approaches to lobectomy.
We therefore decided to review our institutional database on thoracoscopic major lung resection and assess the comorbidity burden of individual patients through the CCI. The purpose of the present study was to evaluate the relationship of an increased comorbidity burden to complications after thoracoscopic lobectomy.
Methods
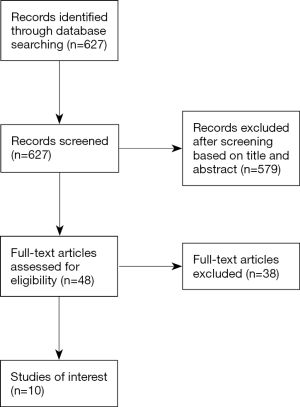
A retrospective study was performed using our institutional database on thoracoscopic major lung resections including retrospective data from 2009 to 2014 and prospective data thereafter. From 2009 through 2016, 609 patients underwent thoracoscopic lobectomy or segmentectomy in our institution. Of those, 512 patients with early stage NSCLC were included in the present study. Excluded were 97 patients operated on for benign disease or pulmonary metastases. This study was approved by the local Institutional Review Board (ID: 019/2016BO2), and specific patient consent was waived.
The overall comorbidity burden was assessed using CCI, which was developed in 1987 and originally included 19 medical conditions exerting substantial impact on survival (1). In the current study, we used the revised version of CCI encompassing 23 medical conditions (Table 1) (15). Compared with the original version, the updated version was found to better predict health outcomes in previous analyses of data from national population health surveys (16). Because lung cancer patients involved in the present study underwent thoracoscopic lobectomies and segmentectomies with curative intent, lung cancer was not scored as malignancy in accordance with previous reports (17). Additionally, a modification was made to score coronary artery disease and myocardial infarction together with a value of 1. Based on the CCI score, the severity of comorbidity burden is usually classified into three grades: mild (CCI score 1–2), moderate (CCI score 3–4) and severe (CCI score ≥5) (18). Accordingly, we considered the comorbidity burden as high grade if CCI score was equal or greater than 3 and low grade if CCI score was less than 3.

Full table
All patients underwent computed tomography, positron emission tomography, and brain magnetic resonance imaging for clinical staging. Those patients with suspected mediastinal nodal metastases were submitted to endobronchial ultrasound guided fine needle aspiration or cervical mediastinoscopy. Lung cancer staging was performed according to the American Joint Committee on Cancer 7th edition manual. Pathologic stage was reported based on the final histopathologic findings after lung resection and systematic mediastinal lymph node dissection.
Physiologic evaluation was undertaken in all patients prior to surgery. Performance status was considered as marginal or poor when Eastern Cooperative Oncology Group scale (ECOG) was equal or higher than 2. Predicted postoperative value of forced expiratory volume in the first second expressed as a percent predicted and of diffusing capacity of lung expressed as a percent predicted (ppoFEV1%, ppoDLCO%) were calculated using the functional segment technique (19). Patients with preoperative lung function testing demonstrating impaired pulmonary function (ppoFEV1% or ppoDLCO% <40) were submitted to stair climb test or cardiopulmonary exercise testing (cycle ergometry) as additional risk stratification prior to lung resection. When performance in stair climb test was not satisfactory, cycle ergometry was used to determine the eligibility. Maximum oxygen consumption higher than 10 mL/kg/min or 35% predicted was considered to be sufficient for major lung resection.
Thoracoscopic lobectomy and segmentectomy were performed using a three-port approach, including a 3-cm anterolateral access incision in the fourth intercostal space without rib spreading and with visualization only through the monitor. Lobar vessels and the bronchus were individually divided. Hilar and mediastinal lymph nodes were dissected.
Mortality was defined as death during the hospitalization for thoracoscopic lobectomy or within 30 days of the operation. Postoperative complications were defined according to the STS/ESTS joint standardization of variable definitions and terminology (3). Pulmonary complications include pneumonia, atelectasis requiring bronchoscopy, adult respiratory distress syndrome, initial ventilator support >48 hours, unplanned re-intubation or tracheotomy. Cardiovascular complications are defined as acute myocardial infarction, pulmonary embolism, and atrial or ventricular arrhythmia requiring intervention. All other adverse events including postoperative bleeding, wound infection, recurrent laryngeal nerve injury, bronchopleural fistula and other relevant events are defined as “other major complications”. Overall morbidity is defined as the occurrence of any major complication including mortality.
Categorical variables were expressed as percentages and evaluated with Fisher’s exact test. Continuous data are reported as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Nearest-neighbor 1:1 propensity score matching without replacement was conducted to control for differences in baseline characteristics between patients with high comorbidity burden and those with low comorbidity burden. To evaluate the causal effect of a high comorbidity burden on postoperative complications, generalized linear mixed model with random effects was fitted. Since matching might introduced a dependency structure, each patient with high comorbidity burden together with the matched counterpart with low comorbidity burden was considered as an individual group and associated with a random intercept in the logistic regression model. We used two-sample comparisons of proportions to calculate the sample size for logistic regression. It was assumed that postoperative complications occured in 25% and 40% of patients with low and high comorbidity burden, respectively (odds ratio =2). Accordingly, we estimated that 200 patients were required for each group to reach a statistical power of 90% at 5% level significance. Statistical significance was declared for P<0.05. Statistical analyses were performed using SPSS, version 22.0 for Windows (SPSS, Chicago, IL, USA). Propensity score matching and analysis using generalized linear mixed model were carried out using R Project for Statistical Computing, Version 3.3.3 along with R package “MatchIt” version 2.4–22 and “lme4” version 1.1–13 (20-22).
Results
There were 228 women and 284 men with a median age of 67 (IQR 61–74) years. The surgical procedures included 480 lobectomies (93.8%) and 32 segmentectomies (6.3%). In 17 patients (3.3%), thoracoscopic procedures had to be converted to thoracotomy. Pathologic analysis demonstrated stage I in 397 (77.7%), stage II in 76 (14.9%) and stage III in 39 patients (7.6%). A high comorbidity burden was found in 193 patients (37.7%), with the remainder of patients having a low comorbidity burden (n=319, 62.3%). The prevalences of each individual comorbid condition are summarized in Table 1. The most common comorbid conditions were hypertension and chronic pulmonary disease, followed by cardiac disease. There were two patients with metastatic solid tumor in their histories. One had breast cancer and a solitary cerebellar metastasis. Another presented with prostate cancer and a bony metastasis. Both patients received curative intent treatments and were disease-free from their prior tumors.
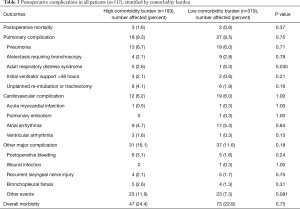
Table 2 categorizes patient demographics, clinical and operative characteristics stratified by comorbidity burden. When compared with their counterparts, patients with a high comorbidity burden more commonly had other risk factors such as increased age, male gender, worse performance status and lower pulmonary function. Postoperative complications are listed in Table 3. Postoperative mortality and morbidity were comparable between patients with high and low comorbidity burdens. There were 5 postoperative deaths for the entire patient cohort. Three patients died from postoperative pneumonia. One patient developed a bronchopleural fistula followed by sepsis and multiple organ failure and died on postoperative day 12. Another developed pneumonia and atrial arrhythmia on postoperative day 5 followed by a bronchopleural fistula, and died on postoperative day 79.

Full table
Full table
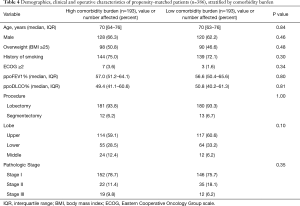
To control for significant differences in baseline characteristics between patients with high and low comorbidity burdens as potential confounders, propensity score matching was performed. The propensity score was assessed using a multivariate logistic regression model which included the following variables: age, gender, ppoFEV1%, ppoDLCO%, and ECOG performance status (≥2 vs. <2). The matching established two well balanced groups each consisting of 193 patients with high or low comorbidity burden (Table 4). After matching, the postoperative complication rates were still comparable between the groups (Table 5). To estimate the causal effect of a high comorbidity burden on postoperative outcomes, analyses of the propensity-matched data were performed using generalized linear mixed model. The results indicated that a high comorbidity burden was not related to greater complication rates after thoracoscopic lobectomy (Table 6).
Full table

Full table

Full table
Discussion
Owing to an ageing population, the presence of multiple comorbid diseases is increasingly common among lung cancer patients (23). Accurate assessment of comorbidity is therefore crucial for determining an optimal treatment strategy, and may be more important than functional assessment in this regard (24). In the present study, we objectively assessed a patient’s overall comorbidity burden using CCI, which considers both number and seriousness of concurrent medical conditions and provides a weighted score for comorbidity (1,24). Interestingly, the incidence of higher comorbidity burden in our patient cohort was greater than that in previously published studies (1,25,26). This discrepancy may be explained by the incorporation of hypertension as the most common comorbid condition into the updated version of CCI used in the current study.
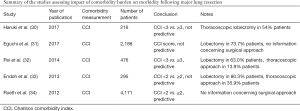
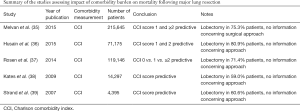
Despite the well established relationship of some major comorbid conditions such as cardiopulmonary and renal diseases to higher postoperative complication rates, the impact of a patient’s overall comorbidity burden on postoperative outcomes following major lung resection has not been thoroughly examined. In a clinical study published in 2003, Birim and colleagues studied 205 patients undergoing lung resections for lung cancer and found that increased comorbidity burden (CCI 3–4) was a strong predictor of major postoperative complications (1). However, the data published in the last 10 years show that the relationship of a higher comorbidity burden to the morbidity after major lung resection is still an object of controversy. While a higher comorbidity burden was found predictive of increased morbidity following lung resection by Rueth et al. and Pei et al., there also have been reports that higher CCI score was not associated with greater postoperative complication rates (6-10). Worthy of notice are substantial selection bias and a wide variety in surgical approaches, extent of resection and cancer stages in these studies. More importantly, none of the recent studies specifically focused on patients undergoing thoracoscopic lobectomy, which has been widely adopted and preferred as surgical approach for treatment of early stage NSCLC (13). Given the positive effect of thoracoscopic approach on postoperative outcomes, it is reasonable to speculate whether a strong relationship still exists between increased comorbidity burden and postoperative morbidity, when lobectomy is performed via a minimally invasive approach.
In the present study, we specifically studied lung cancer patients who underwent thoracoscopic lobectomies in our institution. The analyses indicate that thoracoscopic lobectomy can be performed with low postoperative mortality and reasonable morbidity in patients with a high comorbidity burden. Our results support previous small case series suggesting that thoracoscopic lobectomy is feasible and safe for this patient group (14). In addition, we found that pulmonary, cardiopulmonary complication rates and overall morbidity were comparable between patients with high and low comorbidity burdens. To take into account differences between the comorbidity groups and limit selection bias, we performed propensity score matching to balance preoperative characteristics in the groups. The analyses of propensity-matched data demonstrated similar results. More importantly, our causal effect analyses using propensity-matched data and generalized linear mixed model indicated that a high comorbidity burden measured by CCI was not related to greater complication rates. Thus, our results suggest that the presence of a high comorbidity burden might not be relevant for determining the eligibility for thoracoscopic lobectomy.
Despite recent advances in chronic disease management and perioperative care, the authors felt that the favourable outcomes in our patients with a high comorbidity burden were, at least in part, attributable to the positive effect of thoracoscopic approach on postoperative outcomes and thorough physiologic evaluation performed before surgery. Yet, we also fully acknowledge the limitations of CCI used as a measure of overall comorbidity burden in the present study. These lie mainly in the fact that CCI was not originally constructed to predict postoperative adverse outcomes (27). Notably, the weight assigned to the individual comorbid conditions in CCI may not reflect their relative importance on the risk of postoperative complications. Moreover, CCI includes some comorbid diseases that may not have an impact on the postoperative adverse events following lung resections, e.g. AIDS, skin ulcer and lymphoma. It is, however, worthy of note that those comorbid conditions were either absent or rare in our patient cohort.
Based on our findings, we suggest that future efforts to improve risk assessment for lung cancer patients with multiple comorbid conditions should be directed towards refined preoperative examination of major comorbid diseases and thorough physiologic evaluation. Recently, the ESTS database committee has presented the EuroLung1 score to predict major complications following anatomic lung resection (28). They analyzed nearly 48 thousand anatomic lung resections and identified eight independent morbidity predictors, which encompass three major comorbid diseases: coronary artery disease, cerebrovascular disease and chronic kidney disease. According to the results of regression analyses, they assigned a score ranging from 1 to 3 points to each predictor. The aggregation of these points results in the EuroLung1 score. Given that the underlying data were derived from a large clinical thoracic surgery database like the ESTS database, EuroLung1 score is supposed to be a reliable instrument of risk stratification prior to major lung resection. More encouragingly, new knowledge has emerged showing that frailty assessment is a reliable tool for predicting postoperative adverse outcomes, as the number of elderly patients with lung cancer is growing rapidly. Compared with CCI, frailty assessment is a multidimensional risk assessment quantified not only by comorbid diseases, but also by physical and cognitive impairments, psychosocial risk factors etc. (29). It will be important to focus future research towards identifying the method of measuring frailty best suited for patients considered for major lung resection.
Besides the known imperfection of CCI, the authors also acknowledge other potential limitations of the present study. First, there was the inherent bias of a retrospective approach. Next, our results are derived from single institution data, which may not fully reflect the clinical scenario elsewhere. Caution should be taken in using our results in decision making for patients in other institutions. Of concern also is the selection bias in the present study. Although propensity score matching has mitigated selection bias to some extent by balancing the known confounders, there might be some unknown confounding variables that influence the results. Despite the imperfect data and limitations known to CCI, our results fill the gap in the literature and may help in improving preoperative patient selection and perioperative risk assessment for this patient population.
In conclusion, our results suggest that thoracoscopic lobectomy can be performed with low mortality and reasonable morbidity in lung cancer patients presenting with multiple comorbid diseases. If technically feasible, it is recommended to use a thoracoscopic approach for anatomic lung resection in this patient population. The presence of a high comorbidity burden measured by CCI may not have a perceptible impact on adverse postoperative outcomes following thoracoscopic lobectomy. Preoperative risk assessment for patients with multiple comorbid diseases should focus on examination of major comorbid diseases and physiologic evaluation.
Full table
Full table
Full table
Acknowledgements
We thank Dr. Mark K. Ferguson in The University of Chicago, Chicago, USA for the thorough editing of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the local Institutional Review Board (ID: 019/2016BO2), and specific patient consent was waived.
References
- Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30-4. [Crossref] [PubMed]
- Brunelli A, Salati M, Rocco G, et al. European risk models for morbidity (EuroLung1) and mortality (EuroLung2) to predict outcome following anatomic lung resections: an analysis from the European Society of Thoracic Surgeons database†,‡. Eur J Cardiothorac Surg 2017;51:490-7. [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [Crossref] [PubMed]
- Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 2016;66:337-50. [Crossref] [PubMed]
- Pei G, Zhou S, Han Y, et al. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis 2014;6:1230-8. [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. [Crossref] [PubMed]
- Haruki T, Yurugi Y, Wakahara M, et al. Simplified comorbidity score for elderly patients undergoing thoracoscopic surgery for lung cancer. Surg Today 2017;47:718-25. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Endoh H, Yamamoto R, Satoh Y, et al. Risk analysis of pulmonary resection for elderly patients with lung cancer. Surg Today 2013;43:514-20. [Crossref] [PubMed]
- Sihoe ADL. The evolution of VATS lobectomy. In: Cardoso P, editor. Topics in thoracic surgery. Intech, Croatia (Rijeka), 2011:181-210.
- Zhang R, Ferguson MK. Video-Assisted versus Open Lobectomy in Patients with Compromised Lung Function: A Literature Review and Meta-Analysis. PLoS One 2015;10:e0124512. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, dicussion 28-29.e1.
- Nakanishi R, Nakagawa M, Tokufuchi H, et al. Video-assisted thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: experience with 111 consecutive patients demonstrating comorbidity. Minerva Chir 2012;67:67-75. [PubMed]
- Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008;61:1234-40. [Crossref] [PubMed]
- Witt EA, Goren A. A comparison of the utility of variants of the charlson comorbidity index (CCI) in predicting patient-reported health outcomes. Abstract in International Society for Pharmacoeconomics and Outcomes Research 2014. Value in Health 2014;17:A194. [Crossref]
- Kastner C, Armitage J, Kimble A, et al. The Charlson comorbidity score: a superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis 2006;9:270-4. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e90S.
- Ho DE, Imai K, King G, et al. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Soft 2011;42:1-28. [Crossref]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/
- Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Soft 2015;67:1-48. [Crossref]
- Grose D, Morrison DS, Devereux G, et al. Comorbidities in lung cancer: prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J 2014;90:305-10. [Crossref] [PubMed]
- Guerra M, Neves P, Miranda J. Surgical treatment of non-small-cell lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2013;16:673-80. [Crossref] [PubMed]
- Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg 2005;28:759-62. [Crossref] [PubMed]
- Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non–small cell lung cancer. J Thorac Cardiovasc Surg 2002;123:280-7. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Brunelli A, Salati M, Rocco G, et al. European risk models for morbidity (EuroLung1) and mortality (EuroLung2) to predict outcome following anatomic lung resections: an analysis from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2017;51:490-7. [PubMed]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722-7. [Crossref] [PubMed]
- Haruki T, Yurugi Y, Wakahara M, et al. Simplified comorbidity score for elderly patients undergoing thoracoscopic surgery for lung cancer. Surg Today 2017;47:718-25. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Pei G, Zhou S, Han Y, et al. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis 2014;6:1230-8. [PubMed]
- Endoh H, Yamamoto R, Satoh Y, et al. Risk analysis of pulmonary resection for elderly patients with lung cancer. Surg Today 2013;43:514-20. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. [Crossref] [PubMed]
- Melvan JN, Sancheti MS, Gillespie T, et al. Nonclinical Factors Associated with 30-Day Mortality after Lung Cancer Resection: An Analysis of 215,000 Patients Using the National Cancer Data Base. J Am Coll Surg 2015;221:550-63. [Crossref] [PubMed]
- Husain ZA, Kim AW, Yu JB, et al. Defining the high-risk population for mortality after resection of early stage NSCLC. Clin Lung Cancer 2015;16:e183-7. [Crossref] [PubMed]
- Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014;98:1953-60. [Crossref] [PubMed]
- Kates M, Perez X, Gribetz J, et al. Validation of a model to predict perioperative mortality from lung cancer resection in the elderly. Am J Respir Crit Care Med 2009;179:390-5. [Crossref] [PubMed]
- Strand TE, Rostad H, Damhuis RA, et al. Risk factors for 30-day mortality after resection of lung cancer and prediction of their magnitude. Thorax 2007;62:991-7. [Crossref] [PubMed]