Balancing the benefits and harms of radiotherapy post-radical prostatectomy
We as urologists continue to struggle with patient selection for radiotherapy in men post-radical prostatectomy (RP). We want to offer those men at higher risk, the best opportunity to avoid metastases and death from prostate cancer, however, we want to avoid the morbidity which can be associated with post-operative radiotherapy. Furthermore, we want to avoid offering radiotherapy to men who have occult systemic disease who may not benefit from radiotherapy to the pelvis, yet may still experience the potentially debilitating side-effects of radiotherapy post-RP (1) (Figure 1). There is, therefore, an ongoing discourse regarding patient selection and timing of radiotherapy in the post-RP setting.
Fossati and colleagues have recently published a multi-institutional retrospective cohort study of men with clinically localised prostate cancer, who were treated with salvage radiotherapy (SRT) for persistent or recurrent prostate-specific antigen (PSA) following RP (2). The authors have stratified patients according to clinical risk factors [post-operative PSA level, Gleason score (GS), and T stage] into different risk categories before evaluating the effect of PSA level at the time of SRT on metastasis free survival (MFS). The authors conclude that the PSA level at the time of SRT has no effect on MFS for men in the very low-risk (post-operative PSA undetectable, GS ≤7, T ≤3a) or very high-risk (persistent elevated PSA after surgery, GS ≥8, and T stage) groups. For patients in other groups there appears to be an increase in MFS when SRT is delivered at PSA level <1 ng/mL. Beyond a PSA of 1 ng/mL there is little change in MFS as the PSA increases. It should be noted that the added reduction in the risk of MFS between a PSA of 0 and 1 ng/mL is around 8% for these groups. Therefore, 12.5 men need SRT at PSA =0 ng/mL, compared to PSA =1 ng/mL in order to prevent one case of metastasis occurring.
The occurrence and natural history of biochemical recurrence (BCR) following RP have been well described with BCR occurring in approximately 30% of patients, of whom 23–34% will develop clinical disease and 6% will die of prostate cancer (3,4). Patients with BCR who were managed with observation alone had an average time to development of metastases of eight years, and an average overall survival of 13 years following BCR. Trock and colleagues found that at 6 years, compared to patients receiving SRT, the excess risk of metastases for patients in the observation group was 18% [number needed to treat (NNT) =5.5] and of death 12% (NNT =8) (5). In the present study the average time from RP to BCR was 28 months, and the overall MFS at eight years was 86%, ranging from 62% for men in the highest risk group, to 98% for those at lowest risk (2).
Trock found however, that benefits of SRT were seen only for patients with a PSA doubling time of less than 6 months, and only if SRT was given within 2 years of BCR (concordant with ‘early’ SRT) (5). In the present study the incremental hazard ratio (HR) for PSA at SRT 1.06 per 0.1 ng/mL, which over a PSA rise of 1.0 ng/mL gives a cumulative HR of 1.79 (2). This finding is consistent with those of King, who described an incremental benefit in relapse free survival of 2.6% for each 0.1 ng/mL of PSA prior to SRT (6). It should be noted, however, that in contrast to stage (≥ pT3b HR =2.65), GS (≥8 HR =8.37) and PSA persistence (HR =4.64), the incremental HR of PSA prior to SRT at levels below 1 is of less significance (2).
Moreover, King found that SRT-dose was also correlated with BCR, showing that the relapse-free survival improved by 2% per Gy, suggesting that a treatment dose above 70 Gy should be administered at the lowest possible PSA level (6). However, with dose escalation up to a median of 76 Gy, the rate of genitourinary morbidity increases with grade 2–3 toxicity increasing to 22% for genitourinary and 8% for gastrointestinal symptoms, respectively at 5 years (7).
It is also worth noting that in the present study, the groups of patients at high risk, i.e., those with a persistent PSA following surgery received their SRT at an average interval of 2 months from surgery, compared to the lower risk groups whose PSA was undetectable following surgery, who received SRT at an interval of between 25–31 months from surgery. The former group should probably therefore be considered separately to the lower risk groups. Earlier is not always better however: Briganti et al. found that there was no difference in cancer control between those treated with ART vs. SRT, and Zafutto et al. found that patients treated with ART suffered from worse continence and erectile dysfunction (ED) compared to those treated with SRT or no RT (8,9).
In contrast to previous studies, which have found an association between pre-operative PSA and the risk of BCR and distant metastasis after SRT, Fossati et al. did not use pre-operative PSA when defining the risk groups in this study (2). Furthermore, surgical margin status was not found to be a significant predictor of distant metastasis (HR:1.02, 95% confidence interval: 0.68–1.52) and was omitted from the risk stratification tree. Previous research has generated mixed results: some have found that patients with a positive margin have greater benefits from salvage RT than those with a negative margin; where as others have found that positive margins are key predictor of BCR after SRT (10-13).
The effect of salvage androgen deprivation therapy (ADT) remains unclear in the present study despite evidence that the addition of hormonal therapy to SRT in men with detectable PSA improves long-term outcomes (14,15). A combination of hormonal therapy in addition to radiotherapy might be advisable for men with higher risk disease, but for men with lower risk disease the addition of hormonal therapy may be of limited benefit (16). The efficacy of post-RP radiotherapy (ART and SRT), and neoadjuvant HT are currently being investigated in a number of prospective RCTs (RADICALS, RAVES, and GETUG 17) and the results are eagerly awaited.
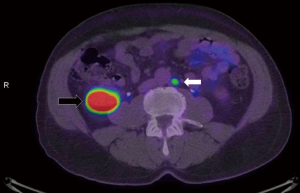
An emerging area of interest not addressed in this study, is the role of advanced imaging in better characterising men with BCR following RP. While conventional imaging such as CT scanning, whole body bone scan, and even MRI scanning have quite poor sensitivity in this setting, it is already apparent that novel molecular imaging such as prostate-specific membrane antigen (PSMA) PET/CT is capable of identifying sites of disease even at very low PSA levels (17,18). In a recent study, van Leeuwen et al. reported their experience of PSMA PET/CT in 70 patients being considered for salvage RT (median PSA 0.2 ng/mL; no patients with PSA >1.0 ng/mL), and observed positive PSMA PET/CT scans in over 50% of patients (19). Of particular note, they reported that (Figure 2) 28.6% of patients had positive scans outside the prostate bed with significant management impact. However, it should also be noted that which patients (if any) might benefit from aggressive approaches to oligometastatic disease identified with novel imaging (20,21).
In conclusion, the current manuscript provides some data on which clinicians can select patients who are likely to benefit from SRT. This selection is important to avoid overtreatment and the associated toxicity both in men at low risk who will not progress to clinical disease, and also in men at very high risk who will have systemic progression of disease and don’t benefit from local salvage therapy. The present study suggests that even in the intermediate groups, the benefits of early SRT are marginal, and the harms of SRT significant. A period of close observation for some months until the PSA approaches 0.5 ng/mL may be reasonable in select patients with intermediate risk features. Even in observation groups, OS can still be acceptable, however, it is important to identify groups at higher risk who will benefit from salvage RT given at an early timepoint. The PSA level following RP, or the BCR that may follow is one factor to consider, along with PSA kinetics, tumour characteristics, patient factors, and novel tools such as PSMA PET/CT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wallis CJ, Glaser A, Hu JC, et al. Survival and Complications Following Surgery and Radiation for Localized Prostate Cancer: An International Collaborative Review. Eur Urol 2018;73:11-20. [Crossref] [PubMed]
- Fossati N, Karnes RJ, Colicchia M, et al. Impact of Early Salvage Radiation Therapy in Patients with Persistently Elevated or Rising Prostate-specific Antigen After Radical Prostatectomy. Eur Urol 2017. [Crossref] [PubMed]
- Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol 2011;59:893-9. [Crossref] [PubMed]
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-7. [Crossref] [PubMed]
- Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760-9. [Crossref] [PubMed]
- King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys 2012;84:104-11. [Crossref] [PubMed]
- Ost P, Lumen N, Goessaert AS, et al. High-dose salvage intensity-modulated radiotherapy with or without androgen deprivation after radical prostatectomy for rising or persisting prostate-specific antigen: 5-year results. Eur Urol 2011;60:842-9. [Crossref] [PubMed]
- Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol 2012;62:472-87. [Crossref] [PubMed]
- Zaffuto E, Gandaglia G, Fossati N, et al. Early Postoperative Radiotherapy is Associated with Worse Functional Outcomes in Patients with Prostate Cancer. J Urol 2017;197:669-75. [Crossref] [PubMed]
- Jia ZW, Chang K, Dai B, et al. Factors influencing biochemical recurrence in patients who have received salvage radiotherapy after radical prostatectomy: a systematic review and meta-analysis. Asian J Androl 2017;19:493-9. [Crossref] [PubMed]
- Kinoshita H, Shimizu Y, Mizowaki T, et al. Risk factors predicting the outcome of salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy. Int J Urol 2013;20:806-11. [Crossref] [PubMed]
- Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 2004;291:1325-32. [Crossref] [PubMed]
- Briganti A, Karnes RJ, Joniau S, et al. Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. Eur Urol 2014;66:479-86. [Crossref] [PubMed]
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017;376:417-28. [Crossref] [PubMed]
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17:747-56. [Crossref] [PubMed]
- Lamb BW, Violet J, Siva S, et al. Re: Radiation With or Without Antiandrogen Therapy in Recurrent Prostate Cancer. Eur Urol 2017;72:320. [Crossref] [PubMed]
- Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016;70:926-37. [Crossref] [PubMed]
- Murphy DG, Hofman M, Lawrentschuk N, et al. Bringing clarity or confusion? The role of prostate-specific membrane antigen positron-emission/computed tomography for primary staging in prostate cancer. BJU Int 2017;119:194-5. [Crossref] [PubMed]
- van Leeuwen PJ, Stricker P, Hruby G, et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int 2016;117:732-9. [Crossref] [PubMed]
- Hicks RJ, Murphy DG, Williams SG. Seduction by Sensitivity: Reality, Illusion, or Delusion? The Challenge of Assessing Outcomes after PSMA Imaging Selection of Patients for Treatment. J Nucl Med 2017;58:1969-71. [Crossref] [PubMed]
- Murphy DG, Sweeney CJ, Tombal B. "Gotta Catch 'em All", or Do We? Pokemet Approach to Metastatic Prostate Cancer. Eur Urol 2017;72:1-3. [Crossref] [PubMed]


