
MLK4 activates the NF-κB network to drive mesenchymal transition in glioblastoma
Glioblastoma (GBM): an invariably lethal diagnosis
Patients diagnosed with malignant glioma (GBM) face a median survival of just 14 months. The standard care following a GBM diagnosis is an aggressive treatment regime that includes surgery, ionizing radiation (IR), and chemotherapy. These treatment strategies provide only palliation and, following the recurrence of the primary tumour, death ultimately ensues. Treatment decisions for GBM are still made based on WHO tumour grading, despite outcomes varying significantly between subgroups. The identification of molecular prognostic markers to guide treatment and target drug therapies to GBM subtypes is therefore critical for improving patient outcomes.
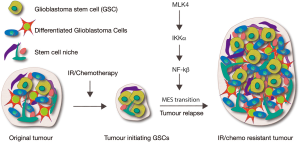
A recent study in Cancer Cell (1) by Lim and colleagues has identified mixed lineage kinase 4 (MLK4) as a novel driver of GBM. In undifferentiated GBMs, high MLK4 expression predicts poorer patient outcome. Moreover, MLK4 promotes radioresistance in mouse GBM models. The authors further demonstrate that MLK4 drives GBM progression by activating the oncogenic nuclear factor kappa B (NF-κB) signalling pathway in glioblastoma stem cells (GSCs), through direct phosphorylation of the NF-κB regulator IKKα (Figure 1). This study therefore provides a new signalling axis for consideration as prognostic marker(s) for tumour aggression and future drug targets required to provide hope for patients with a GBM diagnosis.
Mesenchymal (MES) identity is fundamental to GBM aggression
GBM comprises a highly heterogeneous tumour population. In particular, the capacity to self-renew in the GBM-initiating cells or GSCs, has been strongly implicated in therapeutic resistance and cancer re-initiation following therapy (2-4) (Figure 1). Epithelial to MES cellular transitions (EMT), have been particularly well described in breast cancer as a key indicator of tumour aggression (5,6). A similar EMT-like phenotypic shift in GBM cells, the proneural (PN) to MES transition (PMT) (7-10), has been associated with advanced malignancy, glioma aggressiveness and poorer patient outcome (8,11). Thus, a major challenge in the GBM field is to generate therapeutics targeting GSCs and/or preventing MES transformation. To do this we need to understand factors that distinguish the prognostically distinct PN and MES GSCs. As GBM patients with the MES GSC signature have considerably poorer prognosis, at least in part as a consequence of the resistance of MES-type cells to irradiation therapy, understanding pathways of radiation resistance is critical for improving patient survival.
MLK4/NF-κB: a new pathway in GBM
Despite PMT being associated with brain tumour recurrence (Figure 1), the molecular mechanisms driving this phenotypic shift in the cancer promoting GSCs that remain following therapy are unclear. Genome-wide, genetic, and genomic profiling have revealed the transcriptome of GBM and provided clues on signalling networks “high-jacked” to drive tumorigenesis and drug/radiotherapy resistance (12-14). A recent study in Cancer Cell (1) has demonstrated overexpression of MLK4 in GSCs with MES, but not PN, identity. Moreover, MLK4 expression inversely correlated with patient prognosis in MES, but not PN GBM.
MLK4 is a relatively poorly characterized serine/threonine kinase, however, genome wide analyses have detected MLK4 mutations in GBM and colorectal cancer (CRC) (12,14,15). These observations implicate MLK4 in tumorigenesis, and the positions of identified mutations in MLK4 suggest they may be activating in nature (16), although the effect on kinase function has not been experimentally tested. In this Cancer Cell manuscript (1), the authors demonstrate MLK4 silencing in MES identity GSCs is associated with reduced self-renewal and MES signature loss. Furthermore, loss of MLK4 inhibited cell migration of both de novo and acquired (radiation-induced) MES GSCs both in vitro and in vivo. Together the reduced capacity to self-renew and migrate following MLK4 depletion likely underlies the reduced tumorigenicity of MES-GSCs. Given migration of cells away from the tumour-centre makes surgical resection impossible, and current GBM radiation/drug treatments ineffective, these observations are highly significant. Specifically, targeting the MLK4-axis might provide a means to inhibit invasion and infiltration of GBM cells into normal brain tissue to greatly improve patient outcome.
Although previous work had demonstrated the oncogenic NF-κB signalling-transcription network drives MES transition and IR-resistance in GSCs (8) (Figure 1), the molecular mechanisms for NF-κB activation in the context of GBM were unknown. Lim et al. (1) provide mechanistic insight into this process; MLK4 binds and phosphorylates the NF-κB regulator IKKα, leading to activation of NF-κB signalling in GSCs. IKKα is thus a direct molecular target of MLK4, promoting NF-κB pathway activation and consequential MES-transformation of GSCs (Figure 1). As IKKα is an MLK4 substrate, targeting the MLK4-driven NF-κB signalling axis could provide a novel therapeutic strategy for GBM patients with an MES signature, which were previously impervious to the standard surgery/radiotherapy/chemotherapy regime.
MLK4 is necessary and sufficient for MES identity of GSCs
To identify protein kinases associated with the MES signature, Lim et al. (1) compared genome-wide expression of 349 kinase-encoding genes from patient-derived, high-grade glioma (HGG) spheres (primary GSC-containing neurosphere cultures, 18 PN- and 12 MES-identity). At the mRNA level, six genes (MLK4, LYN, MST4, VRK2, PRKCH, and MAPK9) were significantly upregulated in MES glioma spheres by 4-fold or more compared with PN spheres. Of these, only silencing of MLK4 specifically induced a cell cycle delay (increased the sub-G1 population) and impaired survival of MES, but not PN, glioma spheres. MLK4 mRNA and protein levels were also elevated in HGG patient-derived glioma spheres. Moreover, co-expression of MLK4 with the MES marker, CD44, in tumour spheres was confirmed using immunofluorescence and fluorescence-activated cell sorting (FACS). Thus, although MLK4 mRNA was abundant in the MES identity self-renewing undifferentiated MES glioma spheres, MLK4 mRNA levels rapidly declined following induction of differentiation. These observations link high MLK4 expression with an undifferentiated phenotype i.e. in line with MLK4 activity being associated with more aggressive tumours.
MLK4 shRNA or knockout via CRISPR/Cas9 inhibited clonal growth of MES (not PN) glioma spheres in vitro via inhibition of proliferation combined with induction of apoptosis. Interestingly, shRNA targeting of the other three MLK family genes (MLK1-3) did not differentially effect PN and MES glioma sphere formation. In vivo silencing of MLK4 in MES glioma spheres also decreased tumour progression, and extended median survival, when propagated in intracranial mouse models in vivo. The MES phenotype enables cell migration and extensive invasion of the surrounding healthy brain tissue; a fundamental property of invariably lethal GBMs. Indeed, therapeutic targeting of adhesion-signalling pathways and the actin cytoskeleton has been proposed as a means of blocking GBM cell invasion (17); although toxicity might be associated with targeting such essential cellular structures. Encouragingly, Lim et al. (1) demonstrated that silencing of MLK4 significantly inhibited movement of individual cells from dissociated MES 83 spheres over microfabrics generated to mimic the fiber-like structures of stroma in vitro.
In accordance with this phenotypic shift, MLK4 knockdown decreased mRNA abundance for a number of MES genes (MET, WT1, BCL2A1, VIM, and SNAI1), and was associated with a global reduction of the MES expression signature (via gene-set enrichment analysis, GSEA). Moreover, overexpression of the kinase active MLK4 (R470C) in PN spheres increased expression of 4 MES genes (WT1, BCL2A1, VIM, and SNAI1). Thus, MLK4 was not only required, but was sufficient, for the expression of core MES markers in GSC spheres. Consistent with this, MLK4 knockdown decreased abundance of the MES cell surface antigen CD44 in MES spheres, while overexpression increased CD44 in PN spheres. Xenograft tumours from MLK4 knockdown MES lines also had reduced expression of the MES cell marker VIM, which is a hallmark of invading GBM cells. Moreover, reduced expression of VIM or SNA1 can inhibit glioma cell migration and invasion (18,19).
MLK4 regulates NF-κB in MES GSCs via IKKα phosphorylation
The data above suggest MLK4 regulates the MES signature and, thus, behavior of this class of gliomas. To gain mechanistic insight the authors considered pathways downstream of MLK4. Previous studies placed MLK1–4 in the mitogen-activated protein kinase (MAPK) family that activate c-Jun amino-terminal kinase (JNK) and p38 (20), but neither ERK, JNK nor phospho-p38 levels were altered in PN or MES glioma spheres. Interestingly, given the link between NF-κB pathway and MES differentiation, MLK4 silencing in glioma spheres was found to reduce phospho-IKKα/β, the kinase required upstream of NF-κB to stimulate pathway activity, and thus NF-κB transcriptional activity in MES 83 spheres (using the NF-κB-responsive element for luciferase assays). MLK4 was found to physically interact with both IKKα/β in MES 83 glioma spheres, however, kinase assays revealed that MLK4 only phosphorylates IKKα. Although siRNA targeting of wild type IKKα suppressed MLK4-mediated NF-κB reporter activity, the NF-κB-responsive element was not greatly attenuated by the kinase dead IKKα mutant (S176A). Together with the considerable overlap between genes enriched following MLK4 silencing and the NF-κB pathway signature from GSEA, these data place MLK4 as upstream of IKKα-NF-κB signalling in MES glioma spheres.
MKL4 kinase function is required for MES in GBM
Mutational profiling of bladder, breast, gastric, melanoma, lung, pancreatic and ovarian cancer, colorectal and brain tumours has identified a number of MLK4 mutations (S322P, R442Q, K494Q, P843S, R553STP, R470C). Overall MLK4 mutation frequency was 3% (9 of 340) in CRC and 2% (2 of 113) in GBM (15,21). Although the effect of these mutations on kinase function has not yet been experimentally tested, their positions within MLK4 suggest they may be gain of function. The authors therefore conducted site-directed mutagenesis to create kinase-dead MLK4 (K151A) mutant clones and demonstrated that the MES properties of GSCs were dependent on MLK4 kinase activity. First, MLK4 (K151A) overexpression inhibited proliferation and self-renewal of MES 83 spheres. Second, this MLK4 inactivity was associated with reduced phospho-IKKα/β and NF-κB reporter activity. Third, the physical interaction between IKKα and MLK4 (K151A) was considerably diminished compared with MLK4-WT, suggesting complex formation is sensitive to MLK4 phosphorylation. Finally, and critically for potential roles of the MLK4-axis in driving glioma progression, in vivo tumour growth was significantly reduced by MLK4 (K151A) overexpression in MES 83 spheres and was able to prolong mouse survival.
Closer inspection of xenografted MLK4 kinase dead-MES glioma sphere tumours revealed a lack of central necrosis, which is significant as this is a key criterion for the lethal diagnosis of grade IV GBM. Moreover, these tumours had reduced immunoreactivity to the MES markers CD44 and VIM, which would be expected to attenuate mobility and invasive potential. Together these data suggest that MLK4 normally activates NF-κB via interaction with, and phosphorylation of, IKKα in MES glioma spheres, and that the catalytic/kinase activity of MLK4 is essential for the associated MES phenotype.
IR therapy sensitises PN GSC-derived brain tumours to MLK4 knockdown
IR treatment of glioma spheres increases MES identity and decreases PN markers and, consistent with this, IR induces PMT in GSCs in vitro (7). Kim et al. (1) demonstrate that IR treatment of PN glioma spheres initially upregulates MLK4 mRNA levels and, at a later time point, decreases the abundance of MES markers. IR treatment of intracranial xenografted PN glioma sphere tumours induced NF-κB activity and prolonged mouse survival. Consistent with the in vitro data, MLK4 silencing was not sufficient to alter growth of the PN tumours. Interestingly given IR-resistance is linked to the MES subtype of GSCs (8), MES markers were strongly induced by IR of the PN glioma spheres, but upregulation of CD44 and VIM was no longer observed upon MLK4 silencing. The combination of MLK4 inhibition with IR treatment also reduced tumour growth rates and prolonged survival in the MES glioma sphere-xenograft mice. Furthermore, MLK4-overexpression ameliorated the extended survival normally observed following IR treatment of mice intracranially injected with PN glioma spheres. Thus, MLK4 is both necessary and sufficient for MES radioresistance in these GBM models.
Clinical relevance: MLK4 correlates with poor survival of MES but not PN GBM
Expression of MLK4, OLIG2 (PN marker), and CD44 (MES marker) in 87 HGG specimens (via immunohistochemistry) revealed strong correlation between MLK4 and CD44 expression, while MLK4 and OLIG2 were mutually exclusive. Intriguingly, in the OLIG2-high HGG patients, MLK4 abundance was not informative for post-surgical patient survival. Rather, high-MLK4 patients displayed significantly shorter survival in the CD44-high patient group. Thus, as a prognostic marker, high MLK4 expression predicts poorer post-surgical survival for patients within the MES subgroup.
Is post-therapeutic PMT of GBM tumours a universal phenomenon?
In contrast to many cancers (e.g., colon, prostate, breast), GBM is usually detected in the clinic without prior presentation with a non-invasive tumour; the exception being relatively rare IDH mutant secondary GBMs. Thus, it is not clear whether GBMs generally initiate as PN tumours and accumulate additional mutations to drive MES identity, motility and invasion. There is some evidence that MES transition (PMT) of a small population of PN GSCs drives post-treatment tumour recurrence (10,22) (Figure 1). The acquisition of an MES cell identity by GSCs will also depend on altered signalling and cell-cell contact from the tumour microenvironment/cancer stem cell niche, however, virtually nothing is known of the molecular changes in the cancer niche that drive MES and GBM progression. The authors speculate that MLK4 activation might not only act intrinsically in GSCs to promote MES identity, but that MLK4/NF-κB could also promote GBM progression cell extrinsically. Intriguingly, TNF-α is associated with the macrophages/microglia that infiltrate the GBM microenvironment (8) and, based on this current study, might provide the extrinsic signals required to activate MLK4/NF-κB for GSC PMT and tumour progression.
Applications for the clinic
As radiotherapy is one of the main treatments for GBM, understanding the molecular changes arising in post-IR tumours is essential for improving patient outcomes. As NF-κB is activated in post-IR tumours and a driver of MES (8) it presents as a logical drug target for GBM. Despite the great strides, we have made in developing targeted therapies for transcription factors (23), there are currently no therapies available directly targeting NF-κB. Moreover, given the role of NF-κB in innate immunity (24), chronic NF-κB inhibition could be detrimental as a chemotherapeutic. However, the study by Lim et al. suggests an indirect approach, i.e., targeting MLK4 upstream of NF-κB signalling to inhibit growth of the MES subtype of GBMs.
The first question to ask if MLK4 is to move forward to the clinic is whether this kinase is druggable? Small molecule inhibitors have been reported for other members of the MLK family, suggesting MLK4 is likely to be druggable. However, given silencing of MLK4, but not MLK1–3, differentially inhibits proliferation and drives apoptosis of MES GBM cells, drugs specifically targeting MLK4 will be essential. Ultimately if specific MLK4 inhibitors can be developed, drug toxicity due to off-target side effects will be a critical consideration. The authors predict minimal toxicity as MLK4 is lowly expressed in the normal brain, but we would also need to consider off-target effects in non-neural tissues. Encouragingly, the pan-MLK inhibitor CEP-1347 is well tolerated, with progression to phase II interventional studies in the context of Parkinson’s disease (25). Thus, the MLK4 component of the IKKα/NF-κB signalling axis has excellent potential as a therapeutic target for GBM.
Acknowledgments
Funding: The authors were supported by funding from the National Health and Medical Research Council of Australia and Cancer Council of Victoria.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.46). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim SH, Ezhilarasan R, Phillips E, et al. Serine/Threonine Kinase MLK4 Determines Mesenchymal Identity in Glioma Stem Cells in an NF-κB-dependent Manner. Cancer Cell 2016;29:201-13. [Crossref] [PubMed]
- Seymour T, Nowak A, Kakulas F. Targeting Aggressive Cancer Stem Cells in Glioblastoma. Front Oncol 2015;5:159. [Crossref] [PubMed]
- Ortensi B, Setti M, Osti D, et al. Cancer stem cell contribution to glioblastoma invasiveness. Stem Cell Res Ther 2013;4:18. [Crossref] [PubMed]
- Lathia JD, Mack SC, Mulkearns-Hubert EE, et al. Cancer stem cells in glioblastoma. Genes Dev 2015;29:1203-17. [Crossref] [PubMed]
- Wu Y, Sarkissyan M, Vadgama JV. Epithelial-Mesenchymal Transition and Breast Cancer. J Clin Med 2016;5:13. [Crossref] [PubMed]
- Sánchez-Tilló E, Liu Y, de Barrios O, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci 2012;69:3429-56. [Crossref] [PubMed]
- Mao P, Joshi K, Li J, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A 2013;110:8644-9. [Crossref] [PubMed]
- Bhat KP, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 2013;24:331-46. [Crossref] [PubMed]
- Halliday J, Helmy K, Pattwell SS, et al. In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc Natl Acad Sci U S A 2014;111:5248-53. [Crossref] [PubMed]
- Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157-73. [Crossref] [PubMed]
- Carro MS, Lim WK, Alvarez MJ, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature 2010;463:318-25. [Crossref] [PubMed]
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008;321:1807-12. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455:1061-8. [Crossref] [PubMed]
- Sintupisut N, Liu PL, Yeang CH. An integrative characterization of recurrent molecular aberrations in glioblastoma genomes. Nucleic Acids Res 2013;41:8803-21. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Marusiak AA, Edwards ZC, Hugo W, et al. Mixed lineage kinases activate MEK independently of RAF to mediate resistance to RAF inhibitors. Nat Commun 2014;5:3901. [Crossref] [PubMed]
- Zhong J, Paul A, Kellie SJ, et al. Mesenchymal migration as a therapeutic target in glioblastoma. J Oncol 2010;2010:430142.
- Qi S, Song Y, Peng Y, et al. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS One 2012;7:e38842 [Crossref] [PubMed]
- Xia M, Hu M, Wang J, et al. Identification of the role of Smad interacting protein 1 (SIP1) in glioma. J Neurooncol 2010;97:225-32. [Crossref] [PubMed]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002;3:663-72. [Crossref] [PubMed]
- Martini M, Russo M, Lamba S, et al. Mixed lineage kinase MLK4 is activated in colorectal cancers where it synergistically cooperates with activated RAS signaling in driving tumorigenesis. Cancer Res 2013;73:1912-21. [Crossref] [PubMed]
- Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012;488:522-6. [Crossref] [PubMed]
- Bywater MJ, Poortinga G, Sanij E, et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell 2012;22:51-65. [Crossref] [PubMed]
- Greten FR, Arkan MC, Bollrath J, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 2007;130:918-31. [Crossref] [PubMed]
- Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007;69:1480-90. [Crossref] [PubMed]


