
The International Network for Evaluating Outcomes (iNeo) of neonates: evolution, progress and opportunities
Introduction
The International Network for Evaluating Outcomes (iNeo) in neonates is an international collaboration of nearly population-based national neonatal networks/datasets including 10 networks from 11 countries: Australia, New Zealand, Canada, Finland, Israel, Japan, Spain, Sweden, Switzerland, Tuscany region of Italy and the UK. The network has been, and continues to be, a powerful platform for applied health services and policy research that is aiming to improve patient-oriented outcomes for very preterm (VPT, born before 32 weeks of gestational age) neonates both in the member countries and globally. Only 1% of all births and 14–20% of all preterm births are VPT neonates; however, the rate of VPT birth is important to public health because of these neonates are at high-risk for mortality and childhood morbidities including cerebral palsy, cognitive delay, blindness and deafness (1,2). In addition, the cost of caring for VPT neonates is high both during initial hospitalization (3) and the lifetime cost of permanent impairments.
Various national and international networks/datasets, such as the Vermont Oxford Network in the USA (4), Canadian Neonatal Network (CNN) (5,6), Neonatal Data Analyses Unit in the UK (7) and Swedish Neonatal Quality Register (SNQ) (8,9) analyse data from their local populations to identify trends in outcomes of VPT neonates and benchmark the performance of centres within each network (2,3,10-15). Although most networks initially reported improvements in the rates of morbidity and mortality, recently CNN and other networks observed stagnant progress or worsening of outcomes (16-19). Even when continued outcome improvement was reported, there remained significant variation in the performance of units within neonatal networks. For example, Draper et al. reported the VPT survival rate varied from 74.8% to 93.2% between units in 10 European regions (20). In addition, the risk-adjusted mortality rate for VPT neonates in an Australia NICU was revealed to be lower than a Scottish NICU (21). However, generalizing results from a few NICUs to the population level risks introducing selection bias that can lead to inaccurate conclusions.
Ten years ago, based on individual reports of outcomes, collaborations between the CNN, the Neonatal Research Network of Japan (NRNJ), the Australian and New Zealand Neonatal Network (ANZNN) and the Swedish Neonatal Quality (SNQ) register (9) were initiated. In the first population-based retrospective comparison, we identified that the composite outcome of mortality or any major morbidity [nosocomial infection (NI), necrotizing enterocolitis (NEC), severe neurological injury, retinopathy of prematurity (ROP), and bronchopulmonary dysplasia (BPD)] was lower in NRNJ than Canada in very low birth weight (VLBW) neonates. In-depth analyses revealed that the CNN had higher rates of NI, NEC, and severe neurological injury whereas NRNJ had higher rates of ROP and BPD (22). While the VPT neonate mortality rate was similar between Canada and Australia/New Zealand, rates of NEC, severe neurological injuries, ROP and BPD were significantly lower in Australia/New Zealand than Canada, while the rates of early-onset sepsis and air leaks were higher and the average length of stay was longer than Canada (23,24).
While the variability in neonatal outcomes between Canada and other countries was clear, the factors contributing to the outcome variability were uncertain. Distinct population characteristics, differences in neonate severity of illness, diverse processes of care or delivery of health care could all contribute to outcome variability. For example, Australia/New Zealand has a significantly lower number of outborn VPT neonates than Canada (23); respiratory therapists play a prominent role in North American institutions, but are rarely present in European countries; and in Canada junior doctors are less likely to do shift work than in Europe and Australia.
Given the myriad of factors that could contribute to outcome variability in between countries, it was important to first characterize these factors then identify specific methods to improve neonatal care in each country. The initial care of VPT neonates is highly complex and is typically delivered in specialized units (60–80% of VPT neonates are initially admitted to tertiary NICUs). When VPT neonates no longer need complex care, they are transferred to Level 2 units for the remainder of their hospitalization. Both the day-to-day cost of caring for each neonate in the NICU and the cumulative lifetime cost of care consume an enormous amount of resources. Collaborative sharing and learning between countries to assess practice variations, provide evidence for evidence-based practices, and monitor practice implementation had the potential to help improve outcomes and reduce health care costs globally. However, enabling rigorous comparisons between countries/networks required an understanding of the context for comparison in each country and a way to standardize data.
These concepts, along with enthusiasm of participating networks/data custodians, led to the establishment of an iNeo collaboration in 2013 with funding support from the Institute of Human Development, Child and Youth Health at the Canadian Institutes of Health Research (25). The underlying premise of iNeo was to collect information from population-based networks/datasets on the outcomes, characteristics, practices and culture of the member sites; evaluate the impact of such variations on outcomes and to identify the best models of health service delivery (incorporating medical and non-medical variations); report back to the units the results of analyses; empower units to embrace implementation of data-linked and evidence-based practice changes and ultimately improve outcomes for VPT neonates both within the iNeo countries and globally. In the sections below, we highlight the aims of iNeo and their fulfilment to date and identify opportunities to both expand this initiative to other countries and improve trajectories of growth and development of VPT neonates.
Development and evolution of iNeo
The initial iNeo collaboration comprised 7 datasets from 8 countries, which included Australia and New Zealand (as one network), Canada, Israel, Japan, Spain, Sweden, and the UK Neonatal Collaborative (26). The directors/custodians of these networks/datasets got together at a face-to-face meeting in 2012 to explore the feasibility of the collaboration and how to operationalize the idea. Conceptualizing iNeo was a smooth process; however, we noted many operationalization challenges. After much discussion, the protocol for this initiative was developed and published (27). Subsequently, we added Finland, Switzerland and the Tuscany region of Italy as full members of iNeo bringing the current total to 10 networks/datasets from 11 countries. There have been 3 full-team face-to-face meetings where the development of a minimum dataset, modification of the dataset, analytical plans and results were discussed. Our initial goal was to combine VPT and VLBW neonates (birthweight <1,500 grams) as our base population because of the variability of population criteria used by the networks. However, following our discussions, iNeo now only includes VPT neonates in order to avoid severely growth restricted neonates of high gestational age (GA) in the dataset. The central coordination center for iNeo houses all data and is located in Toronto, Ontario, Canada. Further exploration is needed to determine whether a concept like distributed-data analyses can be applied to allow other countries to have data access.
Aims and achievements of iNeo
The collaboration was built with several aims, which we highlight here while describing what iNeo has achieved and what is planned for the future.
Aim 1: compare national neonatal outcomes and health service organization for VPT neonates
National comparisons of neonatal outcomes and health service organizations have been the primary aim of the iNeo from the outset. The strategy stemmed from a group of studies that each reported comparisons between network outcomes of two countries (22-24,27,28) and culminated in the first ever comparison of outcomes from 9 countries (26). Our key finding was that there are marked variations in adverse outcome rates between countries and none of the participating countries has the best outcomes for all morbidities. A composite outcome of neonatal mortality or morbidities varied from 26% to 42% across countries; however, when confounders were adjusted, two countries had higher standardized rates, two countries had no difference in standardized rates and four countries had lower standardized rates than the other countries (26). We speculated that these differences could be a result of population characteristics, data collection mechanisms, societal factors, healthcare organization factors, cultural factors and practice differences. Untangling composite outcome factors further, we assessed differences in mortality among neonates born at 24–30 weeks’ GA and reported that the survival rate at specific GAs varied between countries; in particular, at 24 weeks’ GA survival varied from ~35% to 84% between countries (29). Similar to mortality, the rates of any ROP varied markedly between networks, especially the rates of treated ROP varied between 4% to 30% between countries (30). Further evaluation of the variation in rates of sepsis (31), NEC (32), and severe brain injury (33) between countries/datasets are underway.
The iNeo database contains information on >200,000 VPT infants, a population size that has allowed us to evaluate the association between exposure to risk factors and neonatal outcomes with a very high precision and especially study rare exposures such as severe congenital heart defects (34). For example, we identified that maternal diabetes was not associated with composite adverse outcome of mortality or major morbidities in VPT neonates (35), and that triplets of <29 weeks’ GA had similar outcomes to singletons of <29 weeks’ GA (36). In contrast, preterm neonates of <29 weeks GA born to mothers with hypertensive disorder were associated with lower odds of mortality, severe brain injury and treated ROP and higher odds of BPD than preterm neonates of <29 weeks GA born to normotensive mothers (37). These associations were, however, highly variable between countries suggesting that we need large datasets to improve the precision of our estimates and the need for network collaborations to generate such large datasets.
Aim 2: identify differences in site-level physical, human, and environmental characteristics, as well as care practices that underlie variations in outcomes
Outcome differences between countries could be a result of variability in care practices as many others have explored; however, the iNeo collaboration hypothesized that the outcome differences could also be a result of site-level physical, human-power, and environmental factors. To identify such variations, a carefully designed, pre-piloted survey with 67 questions (needing 35–45 minutes to complete) was circulated to 390 institutions participating in iNeo. The survey asked about clinical care practices as well as unit-level physical, human, and environmental characteristics as they were in the year 2015. The respondents were also instructed to reply based on unit-level practices and not personal preference. The survey was circulated in 2016 and closed after 3 rounds of reminders. None of the questions were mandatory. A total of 329 (84%) units responded to the survey making this a very high-response survey. We identified marked variations in how perinatal-neonatal services are organized between countries participating in iNeo (38), despite the fact that most of the countries have a publicly-funded healthcare system.
The differences identified in various surveys can explain some of differences in outcomes; however, some of the variability merely reflects different ways of providing care. There was variations identified in the respiratory management of neonates of 23–29 weeks’ GA who were on continuous positive airway pressure support and receiving 30–39% oxygen with some units tolerate high oxygen need and continue CPAP, whereas some units utilize practice of intubate, administer surfactant and extubate (39). It is conceivable that this could explain the variations in the BPD rate between countries (40). In order to understand variations in BPD and ROP, the iNeo survey asked about oxygen saturation limits and determined that >60% of units recently increased their oxygen saturation targets (41) based on recent trial results. The survey also evaluated other practice variations including probiotic use, feeding practices and other risk factors of NEC (42); approach to redirecting care of critically-ill neonates (43); delivery-room deaths across countries (44); screening and treatment criteria for ROP (41); and preventative measures for severe brain injury (45). Finally, we surveyed the physical layout, facilities available, visiting policies (46), and staffing available (47) and identified significant variations between units and countries.
Our next phase of understanding the reasons for differences in outcomes is by linking the survey responses to actual practices and outcomes from individual units and to understand whether any of the practice differences are associated with outcomes or not. These analyses are ongoing and preliminary results are hypothesis generating. We identified that there is marked variations in implementation of proposed NEC prevention practices; however, there was no relationship of implementation of these practices with unit rates of surgical NEC (48). More work is needed to continue evaluating changes in practices and factors associated with improvement in outcomes.
Aim 3: identify clinical and organizational practice improvements relevant to each network
Prior to our multi-country outcomes comparison, individual countries had no idea as to how their outcomes compared with other countries. The results of our first multi-country comparison generated a lot of interest among the participants, which led to several teleconferences and face-to-face meetings to exchange ideas, practices and unit organization and identify potential improvements for local implementation. Several teams of iNeo investigators have visited other collaborating countries to learn and share their knowledge.
More recently, we investigated changes in outcome trends between 2007 and 2015 in iNeo countries to identify areas for improvement. Consistent outcome improvement was observed in a few countries, a static trend in some countries and worsening outcomes in others (49). The differences in outcome trends were hypothesized to be the result of similar factors as described above; however, this challenged us to think beyond those factors as trend evaluation within a country implies those factors being constant.
Aim 4: implement and continually evaluate the impact of data-informed and evidence-linked clinical and organizational practice changes in NICUs within participating networks
Identification of outcomes trends in iNeo countries enabled us to focus first on identifying harmonized definitions for commonly used terminologies in neonates. In collaborations with the International Neonatal Consortium and e-Newborn initiative, a white paper has been produced identifying the need for harmonized definitions. We are also engaged in further work for standardization of terminology (50). A second step will be to develop a data-informed method of identifying clinical and organizational practices that may be associated with improved efficiency and outcomes. This work has only just begun in a few countries; however, discussions are ongoing.
Aim 5: train and mentor junior researchers in the conduct of Neonatal-Perinatal health services research
Training and mentoring junior researchers have been key objectives of iNeo collaboration. Since the inception of iNeo, we have trained 2 post-doctoral fellows, 3 doctoral candidates, 1 master’s level researcher, 2 master’s candidates, 3 post-MD fellows and 1 pre-medical student who all have been successful in completing one project each and publishing it in reputable journals. The training has been open to international trainees and more trainees will be accommodated as we continue to accrue more data.
iNeo research methods in brief
Participation
The following neonatal networks are currently participating in the iNeo collaboration: Australian and New Zealand Neonatal Network (ANZNN, 29 units), Canadian Neonatal Network (CNN, 30 units), Finnish Medical Birth Register (FinMBR, 5 units), Israeli Neonatal Network (INN, 27 units), Neonatal Research Network of Japan (NRNJ, 73 units), Spanish Neonatal Network (SEN1500, 46 units), Swedish Neonatal Quality register (SNQ, 37 units), Swiss Neonatal Network (SNN, 9 NICU), Tuscany region of Italy (TIN Toscane on-line, 4 units) and the UK Neonatal Collaborative (UKNC, 131 units) (Table 1, Appendix 1). All the participating networks collect, analyze, and benchmark the performance and outcomes of their units. Furthermore, to obtain robust population-based estimates, we carefully avoided networks that only included highly specialized units.
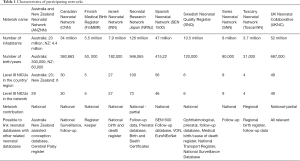
Full table
Development of a minimum dataset
A minimum dataset was created at the iNeo planning meeting in July, 2012 after a detailed review of all the data items collected the eight original participating networks—the elements common to all networks (e.g., gestational age, birth weight, sex, etc.) were selected for the minimum dataset (Appendix 2). Variations in definitions were harmonized for inclusion in the minimum database and were mapped to the International Classification of Diseases and Related Health Problems (ICD-10) (51) and Systematized Nomenclature of Medicine (SNOMED) (52) dictionaries. To ensure consistency and facilitate comparisons over time, some networks redefined their original data formats as part of an ongoing process, while other networks extract data from their databases following the iNeo definitions. Currently, all networks/countries have provided data from 2007–2016 to the coordinating center. The collection items were increased recently to reflect changes in care provision to VPT neonates. New data will only include data from 2017.
Outcomes
The main outcomes of interest for the iNeo collaboration are the following:
- Mortality: death prior to discharge from NICU;
- Severe neurological injury (53): defined as intraventricular hemorrhage with ventricular dilatation, or parenchymal injury [including periventricular leukomalacia (PVL) with or without intraventricular hemorrhage];
- Stage 2 or 3 NEC (54): based on modified Bell’s criteria;
- Severe ROP (55): defined as stage 3 or 4 ROP, or need for laser surgery, or intraocular injections of anti-vascular endothelial growth factor agents;
- NI (56): defined as culture proven sepsis (blood or cerebrospinal fluid positive for pathogenic organism) after 2 days of postnatal age;
- BPD (57): defined as oxygen requirement at 36 weeks post-menstrual age.
These morbidities are associated with an increased risk of long-term severe neurodevelopmental impairment (odds ratios of 1.5 to 3.0) (58). Various studies have shown these morbidities to be predictors of long-term adverse outcomes (59). We are also interested in other outcomes such as patent ductus arteriosus requiring surgical ligation, receipt of delivery room cardiopulmonary resuscitation, air leak syndrome, and resource utilization (length of stay and length of respiratory support).
Analytical framework
- Descriptive analyses of baseline factors: the distribution of neonate characteristics and country-level broad organizational structural features are summarized as counts and percentages for categorical variables using the mean and standard deviation, or the median and interquartile range for continuous variables, and compared among all networks using the Chi-square test for categorical and ANOVA F-test or Mood’s median test for continuous variables.
- Statistical comparisons of outcomes between networks: for the adverse outcomes analyses, initial crude rates and associated 95% and 99% confidence intervals are calculated and graphically displayed using ‘caterpillar plots’ to visually identify differences between networks. To adjust for multiple baseline characteristics, standardized mortality/morbidity ratios (SMRs) are computed using the ‘indirect standardization’ approach. Each network’s observed rate is compared with the expected rate based on the total sample from all other networks to identify networks with rates significantly above or below average. For each outcome, the expected number of events is computed as the sum of predicted probabilities from a multivariable model (logistic regression or zero inflated negative binomial models based on data distribution) adjusted for confounders. Network SMR are graphically displayed using ‘funnel’ plots with 95% and 99% prediction intervals for comparison between networks. In addition, pair-wise comparisons between specific networks with high- and low-outcome rates are performed using multivariate models adjusted for confounders. Statistical models employ generalized estimating equations to adjust analyses for clustering of neonates within networks and for multiples. Statistical significance is evaluated by applying a Bonferroni correction to account for multiple pair-wise comparisons.
Program administration
The day-to-day management of the iNeo collaboration is overseen by the iNeo Director while a Steering Committee comprising of one or two members from each country assesses the overall progress of iNeo, evaluates the scientific merits of proposed projects, reviews results, identifies and articulates strengths and limitations of analyses, and recruits and trains junior researchers interested in international neonatal health. The Director is supported by the iNeo Program Coordinator and a dedicated statistician. The iNeo Coordinating Centre is housed at the Maternal-Infant Care Research Centre (MiCare) within the Samuel Lunenfeld Research Institute at Mount Sinai Hospital, Toronto. Each national data coordinating centre is responsible for local data processing, extraction, transfer, and dissemination of findings to their respective sites.
Financial support for the iNeo coordinating center is provided by an Applied Research Chair Grant from the IHDCYH, CIHR and the infrastructure of individual networks is supported by their own budgets (Appendix 3). The individual network coordinating centres also act as local training sites for trainees in Health Services Research in Neonatal-Perinatal Medicine. In order to foster a true international collaboration, the data collected and housed at the iNeo Coordinating Centre are available to all iNeo member countries and iNeo-affiliated investigators. The policies and procedures governing the transfer of data between national data centres are guided by specific data sharing agreements signed by all participants and their institutes (Appendix 4).
All networks/data custodians have obtained ethics/regulatory approval or its equivalent from their local granting agencies to allow for de-identified data to be collated and sent to the iNeo Coordinating Centre. Overall coordination of the project is also approved by the Research Ethics Board at the Mount Sinai Hospital, Toronto, Ontario Canada for the development, compilation, hosting and management of the iNeo dataset at the MiCare Research Centre. Privacy and confidentiality are of utmost iNeo importance to the iNeo collaboration, and as such data is handled in accordance with the Privacy Commissioner’s guidelines.
Manuscripts published as a result of projects arising from the iNeo collaboration follow the iNeo publication principles (Appendix 5), and all publications have “on behalf of iNeo” as the final author. The remaining publication principles govern data requests and approval, data transfer and analyses, and approval of manuscripts and publications.
Lessons learned
The evolution of iNeo has taught us all critical lessons about the establishment of international collaborations; similarities and differences in healthcare systems, resources, and operations; and variations in outcomes including the following:
- Working with the legal or contracts office at each data hosting institution is critical to overcome systems-associated challenges when establishing international collaborations;
- Privacy and confidentiality are of utmost importance when handling healthcare related data, and it is essential to follow all necessary steps to protect the information;
- Collaborative approaches involving all participating parties can lead to a very fruitful harmonization;
- Everyone should be willing to accept their deficiencies, learn from others and teach others at the same time;
- Allowing data access to all participating countries has many administrative hurdles, including financial support; however, when the processes are incorporated into the collaborative model it facilitates the data sharing process;
- Large collaborations require buy-in from local units; in particular, local units need to understand the contribution of their data to the greater good and the importance of access to large data pools for answering questions with a higher degree of certainty.
Future plans
Future plans for iNeo include the following:
- Expansion of network: the success of iNeo has sparked the interest of several other countries who would like to participate as well. Norway, Taiwan, South Korea, and California in the USA have all expressed interest discussions have begun to potentially add them to the collaboration. In addition, one large unit in Singapore and two in Hong Kong are currently submitting data to the Australian and New Zealand Neonatal Network (ANZNN). More Singapore and Hong Kong units are anticipated to contribute data from 2020. Given the global interest in iNeo, we are expecting that the collaboration will expand to include more countries over the next 2 years.
- Harmonization of data collection across networks: members of iNeo are collaborating with the International Neonatal Consortium (INC) to harmonize data collection of common neonatal conditions, demographic characteristics and diagnoses. The plan is to develop measurable and impactful criteria for use in benchmarking, quality improvement activities and research over the next 2 years. Participants of iNeo have published papers on the harmonization of definitions for both BPD (40) and NEC (60), and we aim to develop recommendations for other diagnoses related to VPT neonates over the next 2 years.
- Dissemination of data collection tools to networks in Low-Middle Income countries: one goal of iNeo is to help develop benchmarking capacity in Low-Middle Income Countries. For this reason, we have shared our minimal database with several NICU in India and China (where 60 units are collecting data using a similar format), which will allow them to benchmark and compare their outcomes among themselves and internationally.
- International neonatal follow-up network: There is a growing interest among iNeo countries to compare 2–3 years neurodevelopmental outcome data for the extremely preterm (<28 weeks GA) neonates. However, networks and countries use different instruments to collect neurodevelopmental outcomes data. To address this problem, iNeo is in the beginning stages of creating a tool to measure the equivalency of outcomes assessed using different instruments. The tool may not result in perfect data harmonization; however, we hope to discover some patterns that can be investigated further.
- Development and execution of network-based clinical trials: to further improve VPT outcomes, high-quality, evidence-informed therapies are needed. However, evidence for new therapies usually comes from large randomized trials, which are time consuming and expensive to conduct. Established networks can facilitate point-of-care trials (61) by linking trial data collection to routine network-level data collection and acting as a conduit for identifying eligible patients, both of which significantly reduce trial cost.
- Collaborative quality improvement activities: the greatest impact any data collection project can have is when data are converted to information and action. For example, multiple networks have reported successful neonatal outcome improvement through data-driven quality improvement efforts (62-66). However, many of the iNeo countries do not have established national/regional quality improvement programs that use their network data. For these networks, the next goal is to learn from networks with established quality improvement programs and use the experience to establish a national/regional quality improvement program in their home countries.
Conclusions
The iNeo collaboration serves as a strong international platform for Neonatal-Perinatal health services research for VPT neonates. The data generated by iNeo continues to provide neonatal benchmarking standards for each country and unit, which will hopefully lead to detailed discussion on how to improve outcomes for VPT neonates globally. Simultaneous capacity building by training junior researchers continues be an added benefit.
Appendix 1 iNeo networks and investigators
ANZNN (Australian and New Zealand Neonatal Network): Kei Lui
CNN (Canadian Neonatal Network): Prakesh S Shah, MD, MSc (Director, Canadian Neonatal Network and site investigator), Mount Sinai Hospital, Toronto, Ontario; Adele Harrison, MD, MBChB, Victoria General Hospital, Victoria, British Columbia; Anne Synnes, MDCM, MHSC, and Joseph Ting, MD, B.C. Women’s Hospital and Health Centre, Vancouver, British Columbia; Zenon Cieslak, MD, Royal Columbian Hospital, New Westminster, British Columbia; Rebecca Sherlock, MD, Surrey Memorial Hospital, Surrey, British Columbia; Wendy Yee, MD, Foothills Medical Centre, Calgary, Alberta; Khalid Aziz, MBBS, MA, MEd, and Jennifer Toye, MD, Royal Alexandra Hospital, Edmonton, Alberta; Carlos Fajardo, MD, Alberta Children’s Hospital, Calgary, Alberta; Zarin Kalapesi, MD, Regina General Hospital, Regina, Saskatchewan; Koravangattu Sankaran, MD, MBBS, and Sibasis Daspal, MD, Royal University Hospital, Saskatoon, Saskatchewan; Mary Seshia, MBChB, Winnipeg Health Sciences Centre, Winnipeg, Manitoba; Ruben Alvaro, MD, St. Boniface General Hospital, Winnipeg, Manitoba; Amit Mukerji, MD, Hamilton Health Sciences Centre, Hamilton, Ontario; Orlando Da Silva, MD, MSc, London Health Sciences Centre, London, Ontario; Chuks Nwaesei, MD, Windsor Regional Hospital, Windsor, Ontario; Kyong-Soon Lee, MD, MSc, Hospital for Sick Children, Toronto, Ontario; Michael Dunn, MD, Sunnybrook Health Sciences Centre, Toronto, Ontario; Brigitte Lemyre, MD, Children’s Hospital of Eastern Ontario and Ottawa General Hospital, Ottawa, Ontario; Kimberly Dow, MD, Kingston General Hospital, Kingston, Ontario; Ermelinda Pelausa, MD, Jewish General Hospital, Montréal, Québec; Keith Barrington, MBChB, Hôpital Sainte-Justine, Montréal, Québec; Christine Drolet, MD, and Bruno Piedboeuf, MD, Centre Hospitalier Universitaire de Québec, Sainte Foy Québec; Martine Claveau, MSc, LLM, NNP, and Marc Beltempo, MD, McGill University Health Centre, Montréal, Québec; Valerie Bertelle, MD, and Edith Masse, MD, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Québec; Roderick Canning, MD, Moncton Hospital, Moncton, New Brunswick; Hala Makary, MD, Dr. Everett Chalmers Hospital, Fredericton, New Brunswick; Cecil Ojah, MBBS, and Luis Monterrosa, MD, Saint John Regional Hospital, Saint John, New Brunswick; Akhil Deshpandey, MBBS, MRCPI, Janeway Children’s Health and Rehabilitation Centre, St. John’s, Newfoundland; Jehier Afifi, MB BCh, MSc, IWK Health Centre, Halifax, Nova Scotia; Andrzej Kajetanowicz, MD, Cape Breton Regional Hospital, Sydney, Nova Scotia; Shoo K Lee, MBBS, PhD (Chairman, Canadian Neonatal Network), Mount Sinai Hospital, Toronto, Ontario.
FinMBR (Finnish Medical Birth Register): Sture Andersson, MD, Helsinki University Hospital, Helsinki; Liisa Lehtonen, MD, Turku University Hospital, Turku; Outi Tammela, MD, Tampere University Hospital, Tampere; Ulla Sankilampi, MD, Kuopio University Hospital, Kuopio; Timo Saarela, MD, Oulu University Hospital, Oulu.
ILL (Illinois Neonatal Network): Preetha Prazad, MD, Advocate Children’s Hospital, Park Ridge, Illinois; Akihiko Noguchi, MD, SSM Cardinal Glennon/St. Mary’s Hospital, St. Louis MO; Kamlesh McWan, MD, Children’s Hospital of Illinois, Peoria, Illinois; Beau Button, MD, St John’s Hospital, Springfield, Illinois; William Stratton, MD, Carle Foundation Hospital, Urbana, Illinois; Aaron Hamvus, MD, Northwestern University Hospitals, Chicago, Illinois; Aarti Raghaven, MD, University Illinois Chicago Hospital, Chicago, Illinois; Matthew Derrick, MD, Evanston Northshore Hospital, Evanston, Illinois; Radley Hadley, MD, Advocate Illinois Masonic Hospital, Chicago, Illinois; Robert Covert, MD, Edward Hospital, Naperville, Illinois; Omar Lablanc, MD, John H. Stroger Cook County Hospital, Chicago, Illinois; Marc Weiss, MD, RMCH Loyola University Hospital, Maywood, Illinois; Anthony Bell, MD, Adventist Hinsdale Hospital, Hinsdale, Illinois; Maliha Shareef, MD, St. Alexius Hospital, Hoffman Estates, Illinois; Jean Silvestri, MD, Rush University Hospital, Chicago, Illinois.
INN (Israel Neonatal Network): Eli Heymann, MD, Assaf Harofeh Medical Center, Tzrifin; Shmuel Zangen, MD, Barzilai Medical Center, Ashkelon; Tatyana Smolkin, MD, Baruch Padeh Medical Center, Poriya; Francis Mimouni, MD, Bikur Cholim Hospital, Jerusalem; David Bader, MD, Bnai Zion Medical Center, Haifa; Avi Rothschild, MD, Carmel Medical Center, Haifa; Zipora Strauss, Chaim Sheba Medical Center, Ramat Gan; Clari Felszer, MD, Emek Medical Center, Afula; Hussam Omari, MD, French Saint Vincent de Paul Hospital, Nazareth; Smadar Even Tov-Friedman, MD, Hadassah University Hospital-Ein Karem, Jerusalem; Benjamin Bar-Oz, MD, Hadassah University Hospital-Har Hazofim, Jerusalem; Michael Feldman, MD, Hillel Yaffe Medical Center, Hadera; Nizar Saad, MD, Holy Family (Italian) Hospital, Nazareth; Orna Flidel-Rimon, MD, Kaplan Medical Center, Rehovot; Meir Weisbrod, MD, Laniado Hospital, Netanya; Daniel Lubin, MD, Mayanei Hayeshua Medical Center, Bnei Brak; Ita Litmanovitz, MD, Meir Medical Center, Kfar Saba; Amir Kugelman, MD, Rambam Medical Center; Eric Shinwell, MD, Rivka Ziv Medical Center, Safed; Gil Klinger, MD, Schneider Children’s Medical Center of Israel, Rabin Medical Center (Beilinson Campus), Petah Tikva; Yousif Nijim, MD, Scottish (EMMS) Hospital, Nazareth; Alona Bin-Nun, MD, Shaare-Zedek Medical Center, Jerusalem; Agneta Golan, MD, Soroka Medical Center, Beersheba; Dror Mandel, MD, Sourasky Medical Center, Tel Aviv; Vered Fleisher-Sheffer, MD,Western Galilee Medical Center, Nahariya; David Kohelet, MD, Wolfson Medical Center, Holon; Lev Bakhrakh, MD, Yoseftal Hospital, Eilat.
NRNJ (Neonatal Research Network Japan): Satoshi Hattori, MD, Sapporo City Hospital, Sapporo, Hokkaido; Masaru Shirai, MD, Asahikawa Kosei Hospital, Asahikawa, Hokkaido; Toru Ishioka, MD, Engaru Kosei Hospital, Engaru, Hokkaido; Toshihiko Mori, MD, NTT East Sappro Hospital, Sapporo, Hokkaido; Takasuke Amizuka, MD, Aomori Prefecture Central Hospital, Aomori, Aomori; Toru Huchimukai, MD, Iwate Prefecture Ohfunato Hospital, Ofunato, Iwate; Hiroshi Yoshida, MD, Tsuruoka City Shonai Hospital, Tsuruoka, Yamagata; Ayako Sasaki, MD, Yamagata University, Yamagata, Yamagata; Junichi Shimizu, MD, Tsuchiura Kyodo Hospital, Tsuchiura, Ibaraki; Toshihiko Nakamura, MD, National Nishisaitama Central Hospital, Tokorozawa, Saitama; Mami Maruyama, MD, Jichi Medical University Saitame Medical Center, Omiya, Saitama; Hiroshi Matsumoto, MD, Asahi Central Hospital, Asahi, Chiba; Shinichi Hosokawa, MD, National International Medical Center, Shinjuku, Tokyo; Atsuko Taki, MD, Tokyo Medical and Dental University, Bunkyo, Tokyo; Machiko Nakagawa, MD, Saint Luku Hospital, Chuo, Tokyo; Kyone Ko, MD, Sanikukai Hospital, Sumida, Tokyo; Azusa Uozumi, MD, Odawara City Hospital, Odawara, Kanagawa; Setsuko Nakata, MD, Iida City Hospital, Iida, Nagano; Akira Shimazaki, MD, National Shinshu Ueda Medical Center, Ueda, Nagano; Tatsuya Yoda, MD, Saku General Hospital, Saku, Nagano; Osamu Numata, MD, Nagaoka Red Cross Hospital, Nagaoka, Niigata; Hiroaki Imamura, MD, Koseiren Takaoka Hospital, Takaoka, Toyama; Azusa Kobayashi, MD, Kanazawa Medical University, Kanazawa, Kanazawa; Shuko Tokuriki, MD, Fukui University, Fukui, Fukui; Yasushi Uchida, MD, National Nagara Medical Center, Nagara, Gifu; Takahiro Arai, MD, Takayama Red Cross Hospital, Takayama, Gifu; Mitsuhiro Ito, MD, Fujieda City Hospital, Fujieda, Shizuoka; Kuniko Ieda, MD, Koritsu Tosei Hospital, Toyota, Aichi; Toshiyuki Ono, MD, Komaki City Hospital, Komaki, Aichi; Masashi Hayashi, MD, Okazaki City Hospital, Okazaki, Aichi; Kanemasa Maki, MD, Yokkaichi City Hospital, Yokkaichi, MieToru Yamakawa, MD, Japan Baptist Hospital, Kyoto, Kyoto; Masahiko Kawai, MD, Kyoto University, Kyoto, Kyoto; Noriko Fujii, MD, Fukuchiyama City Hospital, Fukuchiyama, Kyoto; Kozue Shiomi, MD, Kyoto City Hospital, Kyoto, Kyoto; Koji Nozaki, MD, Mitubishi Kyoto Hospital, Kyoto, Kyoto; Hiroshi Wada, MD, Yodogawa Christian Hospital, Osaka, Osaka; Taho Kim, MD, Osaka City Sumiyoshi Hospital, Osaka, Osaka; Yasuyuki Tokunaga, MD, Toyonaka City Hospital, Toyonaka, Osaka;, MD, National Cerebral and Cardiovascular Center, Suita, Osaka; Akihiro Takatera, MD, Chifune Hospital, Osaka, Osaka; Toshio Oshima, MD, Bell Land General Hospital, Sakai, Osaka; Hiroshi Sumida, MD, Rinku General Hospital, Izumisano, Osaka; Yae Michinomae, MD, Yao City Hospital, Yao, Osaka; Yoshio Kusumoto, MD, Osaka General Medical Center, Osaka, Osaka; Seiji Yoshimoto, MD, Kobe Children’s Hospital, Kobe, Hyogo; Takeshi Morisawa, MD, Kakogawa City Hospital, Kakogawa, Hyogo; Tamaki Ohashi, MD, Hyogo Prefectural Awaji Hospital, Sumoto, Hyogo; Yukihiro Takahashi, MD, Nara Prefecture Medical University, Kashiwara, Nara; Moriharu Sugimoto, MD, Tsuyama Central Hospital, Tsuyama, Okayama; Noriaki Ono, MD, Hiroshima University, Hiroshima, Hiroshima; Shinichiro Miyagawa, MD, National Kure Medical Center, Kure, Hiroshima; Takahiko Saijo, MD, Tokushima University, Tokushima, Tokushima; Takashi Yamagami, MD, Tokushima City Hospital, Tokushima, Tokushima; Kosuke Koyano, MD, Kagawa University, Kida, Kagawa; Shoko Kobayashi, MD, Shikoku Medical Center for Children and Adults, Zentsuji, Kagawa; Takeshi Kanda, MD, National Kyushu Medical Center, Fukuoka, Fukuoka; Yoshihiro Sakemi, MD, National Kokura Medical Center, Kitakyushu, Fukuoka; Mikio Aoki, MD, National Nagasaki Medical Center, Nagasaki, Nagasaki; Koichi Iida, MD, Oita Prefectural Hospital, Oita, Oita; Mitsushi Goshi, MD, Nakatsu City Hospital, Nakatsu, Oita; Yuko Maruyama, MD, Imakyure General Hospital, Kagoshima, Kagoshima.
SEN1500 (Spanish Neonatal Network): Alejandro Avila-Alvarez, MD, and José Luis Fernandez-Trisac, MD, Complexo Hospitalario Universitario De A Coruña, A Coruña; Mª Luz Couce Pico, MD, and María José Fernández Seara, MD, Hospital Clínico Universitario de Santiago, Santiago de Compostela; Andrés Martínez Gutiérrez, MD, Complejo Hospitalario Albacete, Albacete; Carolina Vizcaíno, MD, Hospital General Universitario de Elche, Alicante; Miriam Salvador Iglesias, MD, and Honorio Sánchez Zaplana, MD, Hospital General Universitario de Alicante, Alicante; Belén Fernández Colomer, MD, and José Enrique García López, MD, Hospital Universitario Central de Asturias, Oviedo, Asturias; Rafael García Mozo, MD, and M. Teresa González Martínez, MD, Hospital Universitario de Cabueñes, Gijón, Asturias; Mª Dolores Muro Sebastián, MD, and Marta Balart Carbonell, MD, Clínica Corachán, Barcelona; Joan Badia Barnusell, MD, and Mònica Domingo Puiggròs, MD, Corporacio Parc Taulí, Sabadell, Barcelona; Josep Figueras Aloy, MD, and Francesc Botet Mussons, MD, Hospital Clínic de Barcelona, Barcelona; Israel Anquela Sanz, MD, Hospitalario De Granollers, Granollers; Gemma Ginovart Galiana, MD, H. De La Santa Creu I Sant Pau, Barcelona; W. Coroleu, MD, Hospital Universitari Germans Trias I Pujol, Badalona; Martin Iriondo, MD, Hospital Sant Joan de Déu Barcelona, Esplugues de Llobregat, Barcelona; Laura Castells Vilella, MD, Hospital General de Cataluña, Barcelona; Roser Porta, MD, Institute Dexeus, Barcelona; Xavier Demestre, MD, and Silvia Martínez Nadal, MD, Scias-Hospital Barcelona, Barcelona; Cristina de Frutos Martínez, MD, Hospital Universitario de Burgos, Burgos; María Jesús López Cuesta, MD, H. San Pedro de Alcántara, Cáceres; Dolores Esquivel Mora, MD, and Joaquín Ortiz Tardío, MD, Hospital Jerez, Cádiz; Isabel Benavente, MD, and Almudena Alonso, MD, Hospital Universitario Puerta Del Mar, Cádiz; Ramón Aguilera Olmos, MD, Hospital General de Castellón, Castellón; Miguel A. García Cabezas, MD, and Mª Dolores Martínez Jiménez, MD, Hospital General Universitario de Ciudad Real, Ciudad Real; Mª Pilar Jaraba Caballero, MD, and Mª Dolores Ordoñez Díaz, MD, Hospital Universitario Reina Sofía, Córdoba; Alberto Trujillo Fagundo, MD, and Lluis Mayol Canals, MD, Hospital Universitari de Girona Dr. Josep Trueta, Girona; Fermín García-Muñoz Rodrigo, MD, and Lourdes Urquía Martí, MD, H.M.I. Las Palmas, Las Palmas, Gran Canaria; María Fernanda Moreno Galdo, MD, and José Antonio Hurtado Suazo, MD, Hospital Universitario Virgen De Las Nieves, Granada; Eduardo Narbona López, and José Uberos Fernández, MD, Hospital Universitario San Cecilio, Granada; Miguel A Cortajarena Altuna, MD, and Oihana Muga Zuriarrain Hospital, MD, Donostia, Gipuzkoa; David Mora Navarro, MD, Hospital Juan Ramón Jiménez, Huelva; María Teresa Domínguez, MD, Hospital Costa De La Luz, Huelva; Mª Yolanda Ruiz del Prado, MD, and Inés Esteban Díez, MD, Hospital San Pedro, Logroño, La Rioja; María Teresa Palau Benavides, MD, and Santiago Lapeña, MD, Hospital de León, León, León; Teresa Prada, MD, Hospital del Bierzo, Ponferrada, León; Eduard Soler Mir, MD, Hospital Arnau De Vilanova, Lleida; Araceli Corredera Sánchez, MD, Enrique Criado Vega, MD, Náyade del Prado, MD, and Cristina Fernández, MD, Hospital Clínico San Carlos, Madrid; Lucía Cabanillas Vilaplana, MD, and Irene Cuadrado Pérez, MD, Hospital Universitario De Getafe, Madrid; Luisa López Gómez, MD, Hospital De La Zarzuela, Madrid; Laura Domingo Comeche, MD, Hospital Universitario de Fuenlabrada, Fuenlabrada, Madrid; Isabel Llana Martín, MD, Hospital Madrid-Torrelodones, Madrid, Madrid; Carmen González Armengod, MD, and Carmen Muñoz Labián, MD, Hospital Universitario Puerta De Hierro, Majadahonda, Madrid; Mª José Santos Muñoz, MD, Hospital Severo Ochoa, Leganés, Madrid; Dorotea Blanco Bravo, MD, and Vicente Pérez, MD, Hospital Gregorio Marañón, Madrid; Mª Dolores Elorza Fernández, MD, Celia Díaz González, MD, and Susana Ares Segura, MD, H.U. La Paz, Madrid; Manuela López Azorín, MD, Hospital Universitario Quirónsalud Madrid, Madrid; Ana Belén Jimenez MD, Hospital Universitario Fundación Jiménez Díaz, Madrid; Tomás Sánchez-Tamayo, MD, and Elías Tapia Moreno, MD, Hospital Carlos Haya, Málaga; María González, MD, and José Enrique Sánchez Martínez, MD, Hospital Parque San Antonio De Málaga, Málaga; José María Lloreda García, MD, Hospital Universitario Santa Lucia De Cartagena, Murcia; Concepción Goñi Orayen, MD, Hospital Virgen Del Camino De Pamplona, Pamplona, Navarra; Javier Vilas González, MD, Complexo Hospitalario Pontevedra, Pontevedra; María Suárez Albo, MD, and Eva González Colmenero, MD, Hospital Xeral De Vigo, Pontevedra; Elena Pilar Gutiérrez González, MD, and Beatriz Vacas del Arco, MD, Hospital Universitario de Salamanca, Salamanca; Josefina Márquez Fernández, MD, and Laura Acosta Gordillo, MD, Hospital Valme, Sevilla; Mercedes Granero Asensio, MD, Hospital Virgen De La Macarena, Sevilla; Carmen Macías Díaz, MD, Hospital Universitario Virgen Del Rocío, Sevilla; Mar Albújar, MD, Hospital Universitari de Tarragona Joan XXIII, Tarragona; Pedro Fuster Jorge. MD, Hospital Universitario De Canarias, San Cristóbal de La Laguna, Santa Cruz de Tenerife; Sabina Romero, MD, and Mónica Rivero Falero, MD, Hospital Universitario Nuestra Señora De Candelaria, Santa Cruz de Tenerife; Ana Belén Escobar Izquierdo, Hospital Virgen De La Salud, Toledo; Javier Estañ Capell, MD, Hospital Clinico Universitario De Valencia, Valencia; Mª Isabel Izquierdo Macián, MD, Hospital Universitari La Fe, Valencia; Mª Mar Montejo Vicente, MD, and Raquel Izquierdo Caballero, MD, Hospital Universitario Río Hortega, Valladolid; Mª Mercedes Martínez, MD, and Aintzane Euba, MD, Hospital de Txagorritxu, Vitoria-Gasteiz; Amaya Rodríguez Serna, MD, and Juan María López de Heredia Goya, MD, Hospital de Cruces, Baracaldo; Alberto Pérez Legorburu, MD, and Ana Gutiérrez Amorós, MD, Hospital Universitario de Basurto, Bilbao; Víctor Manuel Marugán Isabel, MD, and Natalio Hernández González, MD, Hospital Virgen De La Concha - Complejo Asistencial De Zamora, Zamora; Segundo Rite Gracia, MD, Hospital Miguel Servet, Zaragoza; Mª Purificación Ventura Faci, MD, and Mª Pilar Samper Villagrasa, MD, Hospital Clínico Universitario Lozano Blesa, Zaragoza.
SNQ (Swedish Neonatal Quality Register): Jiri Kofron, MD, Södra Älvsborgs Sjukhus, Borås; Katarina Strand Brodd, MD, Mälarsjukhuset, Eskilstuna; Andreas Odlind, MD, Falu Lasarett, Falun; Lars Alberg, MD, Gällivare Sjukhus, Gällivare; Sofia Arwehed, MD, Gävle Sjukhus, Gävle; Ola Hafström, MD, SU/Östra, Göteborg; Anna Kasemo, MD, Länssjukhuset, Halmstad; Karin Nederman, MD, Helsingborgs Lasarett, Helsingborg; Lars Åhman, MD, Hudiksvalls Sjukhus, Hudiksvall; Fredrik Ingemarsson, MD, Länssjukhuset Ryhov, Jönköping; Henrik Petersson, MD, Länssjukhuset, Kalmar; Pernilla Thurn, MD, Blekingesjukhuset, Karlskrona; Eva Albinsson, MD, Centralsjukhuset, Karlstad; Bo Selander, MD, Centralsjukhuset, Kristianstad; Thomas Abrahamsson, MD, Universitetssjukhuset, Linköping; Ingela Heimdahl, MD, Sunderby sjukhus, Luleå; Kristbjorg Sveinsdottir, MD, Skånes Universitetssjukhus, Malmö/Lund; Erik Wejryd, MD, Vrinnevisjukhuset, Norrköping; Anna Hedlund, MD, Skellefteå Lasarett, Skellefteå; Maria Katarina Söderberg, MD, Kärnsjukhuset Skaraborg, Skövde; Boubou Hallberg, MD, Karolinska Sjukhuset, Stockholm; Thomas Brune, MD, Södersjuhuset, Stockholm; Jens Bäckström, MD, Länssjukhuset, Sundsvall; Johan Robinson, MD, Norra Älvsborgs Länssjukhus, Trollhättan; Aijaz Farooqi, MD, Norrlands Universitetssjukhus, Umeå; Erik Normann, MD, Akademiska Barnsjukhuset, Uppsala; Magnus Fredriksson, MD, Visby Lasarett, Visby; Anders Palm, MD, Västerviks Sjukhus, Västervik; Urban Rosenqvist, MD, Centrallasarettet, Västerås; Bengt Walde, MD, Centrallasarettet, Växjö; Cecilia Hagman, MD, Lasarettet, Ystad; Andreas Ohlin, MD, Universitetssjukhuset, Örebro; Rein Florell, MD, Örnsköldsviks Sjukhus, Örnsköldsvik; Agneta Smedsaas-Löfvenberg, MD, Östersunds Sjukhus, Östersund.
Swiss NeoNet (Switzerland Neonatal Network): Philipp Meyer, MD, and Claudia Anderegg, MD, Cantonal Hospital, Children’s Clinic, Aarau; Sven Schulzke, MD, University Children’s Hospital, Basel; Mathias Nelle, MD, University Hospital, Berne; Bendicht Wagner, MD, University Hospital, Berne; Thomas Riedel, MD, Children’s Hospital, Chur; Grégoire Kaczala, MD, Cantonal Hospital, Fribourg; Riccardo E. Pfister, MD, University Hospital (HUG), Geneva; Jean-François Tolsa, MD, and Matthias Roth, MD, University Hospital (CHUV), Lausanne; Martin Stocker, MD, Children’s Hospital, Lucerne; Bernhard Laubscher, MD, Cantonal Hospital, Neuchatel; Andreas Malzacher, MD, Cantonal Hospital, St. Gallen; John P. Micallef, MD, Children’s Hospital, St. Gallen; Lukas Hegi, MD, Cantonal Hospital, Winterthur; Dirk Bassler, MD, and Romaine Arlettaz, MD, University Hospital (USZ), Zurich; Vera Bernet, MD, University Children’s Hospital, Zurich.
TIN Toscane on-line Network: Carlo Dani, MD, Careggi University Hospital, Florence, Italy; Patrizio Fiorini, MD, Anna Meyer Children’s University Hospital, Florence, Italy; Antonio Boldrini, MD, University Hospital of Pisa, Pisa, Italy; Barbara Tomasini, MD, University Hospital of Siena, Siena, Italy.
UKNC (UK Neonatal Collaborative): Anita Mittal, MBChB, Bedford Hospital, Bedford, Bedfordshire; Jonathan Kefas, MBChB, Lister Hospital, Stevenage, Hertfordshire; Anand Kamalanathan, MBChB, Arrowe Park Hospital, Wirral, Merseyside; Jayachandran, MBChB, Leighton Hospital, Crewe, Cheshire; Bill Yoxall, MBChB, Liverpool Women’s Hospital, Liverpool, Merseyside; Tim McBride, MBChB, Ormskirk District General Hospital, Ormskirk, Lancashire; Delyth Webb, MBChB, Warrington Hospital, Warrington, Cheshire; Ross Garr, MBChB, Whiston Hospital, Prescot, Merseyside; Ahmed Hassan, MBChB, Broomfield Hospital, Chelmsford, Essex; Priyadarshan Ambadkar, MBChB, James Paget Hospital, Gorleston, Norfolk; Mark Dyke, MBChB, Norfolk & Norwich University Hospital, Norwich, Norfolk; Katharine McDevitt, MBChB, Peterborough City Hospital, Peterborough, Cambridgeshire; Glynis Rewitzky, MBChB, Queen Elizabeth Hospital, King’s Lynn, Birmingham, West Midlands; Angela D’Amore, MBChB, Rosie Maternity Hospital, Addenbrookes, Cambridge, Cambridgeshire; Nagesh Panasa, MBChB, North Manchester General Hospital, Manchester, Greater Manchester; Paul Settle, MBChB, Royal Bolton Hospital, Bolton, Lancashire; Natasha Maddock, MBChB, Royal Oldham Hospital, Manchester, Greater Manchester; Ngozi Edi-Osagie, MBChB, St Mary’s Hospital, Manchester, Greater Manchester; Christos Zipitis, MBChB, The Robert Albert Edward Infirmary, Wrightington, Greater Manchester; Carrie Heal, MBChB, Stepping Hill Hospital, Stockport, Cheshire; Jacqeline Birch, MBChB, Tameside General Hospital, Ashton-under-Lyne, Lancashire; Abdul Hasib, MBChB, Darent Valley Hospital, Dartford, Kent; Aung Soe, MBChB, Medway Maritime Hospital, Gillingham, Kent; Niraj Kumar, MBChB, Queen Elizabeth The Queen Mother Hospital, Margate, Kent; Hamudi Kisat, MBChB, Tunbridge Wells Hospital, Tunbridge Wells, Kent; Vimal Vasu, MBChB, William Harvey Hospital, Ashford, Kent; Meera Lama, MBChB, Lancashire Women & Newborn Centre, Burnley, Lancashire; Richa Gupta, MBChB, Royal Preston Hospital, Preston, Lancashire; Chris Rawlingson, MBChB, Victoria Hospital, Blackpool, Blackpool, Lancashire; Tim Wickham, MBChB, Barnet Hospital, Barnet, Hertfordshire; Marice Theron, MBChB, The Royal Free Hospital, Hampstead, London; Giles Kendall, MBChB, University College Hospital, Fitzrovia, London; Aashish Gupta, MBChB, Basildon Hospital, Basildon, Essex; Narendra Aladangady, MBChB, Homerton Hospital, Hackney, London; Imdad Ali, MBChB, Newham General Hospital, Newham, London; Lesley Alsford, MBChB, North Middlesex University Hospital, Edmonton, London; Wilson Lopez, MBChB, Queen’s Hospital, Romford, Essex; Vadivelam Murthy, MBChB, The Royal London Hospital, Whitechapel, London; Caroline Sullivan, MBChB, Whipps Cross University Hospital, Whipps Cross, London; Mark Thomas, MBChB, Chelsea & Westminster Hospital, Chelsea, London; Tristan Bate, MBChB, Hillingdon Hospital, Hillingdon, London; Sunit Godambe, MBChB, Queen Charlotte’s Hospital, East Acton, London; Sunit Godambe, MBChB, St Mary’s Hospital, Westminister, London; Timothy Watts, MBChB, Guy’s & St Thomas’ Hospital, Lambeth, London; Jauro Kuna, MBChB, University Hospital Lewisham, Lewisham, London; John Chang, MBChB, Croydon University Hospital, Croydon, Surrey; Vinay Pai, MBChB, Kingston Hospital, Kingston, London; Charlotte Huddy, MBChB, St George’s Hospital, Wandsworth, London; Salim Yasin, MBChB, St. Helier Hospital, Merton, London; Richard Nicholl, MBChB, Northwick Park Hospital, Brent, London; Poornima Pandey, MBChB, Kettering General Hospital, Kettering, Northhamptonshire; Jonathan Cusack, MBChB, Leicester General Hospital, Leicester, Leicestershire; Venkatesh Kairamkonda, MBChB, Leicester Royal Infirmary, Leicester, Leicestershire; Dominic Muogbo, MBChB, Queen’s Hospital, Burton On Trent, Burton-on-Trent, Staffordshire; Liza Harry, MBChB, Alexandra Hospital, Redditch, Worcestershire; Phil Simmons, MBChB, Birmingham Heartlands Hospital, Birmingham, West Midlands; Julie Nycyk, MBChB, City Hospital, Birmingham, West Midlands; Phil Simmons, MBChB, Good Hope Hospital, Birmingham, West Midlands; Andrew Gallagher, MBChB, Worcestershire Royal Hospital, Worcester, Worcestershire; Tilly Pillay, MBChB, New Cross Hospital, Wolverhampton, West Midlands; Sanjeev Deshpande, MBChB, Royal Shrewsbury Hospital, Shrewsbury, Shropshire; Mahadevan, MBChB, Russells Hall Hospital, Dudley, West Midlands; Alison Moore, MBChB, University Hospital of North Staffordshire, Hartshill, Staffordshire; Simon Clark, MBChB, The Jessop Wing, Sheffield, South Yorkshire; Mehdi Garbash, MBChB, Darlington Memorial Hospital, Darlington, County Durham; Mithilesh Lal, MBChB, James Cook University Hospital, Middlesborough, North Yorkshire; Majd Abu-Harb, MBChB, Sunderland Royal Hospital, Sunderland, Tyne and Wear; Mehdi Garbash, MBChB, University Hospital Of North Durham, Durham, Durham; Alex Allwood, MBChB, Derriford Hospital, Plymouth, Devon; Michael Selter, MBChB, North Devon District Hospital, Barnstaple, Devon; Paul Munyard, MBChB, Royal Cornwall Hospital, Truro, Cornwall; David Bartle, MBChB, Royal Devon & Exeter Hospital, Exeter, Devon; Siba Paul, MBChB, Torbay Hospital, Torquay, Devon; Graham Whincup, MBChB, Conquest Hospital, St.Leonards-on-sea, East Sussex; Abdus Mallik, MBChB, Frimley Park Hospital, Frimley, Surrey; Philip Amess, MBChB, Princess Royal Hospital, Telford, Shropshire; Charles Godden, MBChB, Royal Surrey County Hospital, Guildford, Surrey; Philip Amess, MBChB, Royal Sussex County Hospital, Brighton, East Sussex; Peter Reynolds, MBChB, St Peter’s Hospital, Chertsey, Surrey; Indranil Misra, MBChB, Milton Keynes Foundation Trust Hospital, Milton Keynes, Buckinghamshire; Peter De Halpert, MBChB, Royal Berkshire Hospital, Reading, Berkshire; Sanjay Salgia, MBChB, Stoke Mandeville Hospital, Aylesbury, Buckinghamshire; Rekha Sanghavi, MBChB, Wexham Park Hospital, Slough, Berkshire; Ruth Wigfield, MBChB, Basingstoke & North Hampshire Hospital, Basingstoke, Hampshire; Abby Deketelaere, MBChB, Dorset County Hospital, Dorchester, Dorset; Minesh Khashu, MBChB, Poole Hospital NHS Foundation Trust, Poole, Dorset; Michael Hall, MBChB, Princess Anne Hospital, Southampton, Hampshire; Charlotte Groves, MBChB, Queen Alexandra Hospital, Portsmouth, Hampshire; Nick Brown, MBChB, Salisbury District Hospital, Salisbury, Wiltshire; Nick Brennan, MBChB, St Richard’s Hospital, Chichester, West Sussex; Katia Vamvakiti, MBChB, Worthing Hospital, Worthing, West Sussex; John McIntyre, MBChB, Royal Derby Hospital, Derby, Derbyshire; Simon Pirie, MBChB, Gloucestershire Royal Hospital, Gloucester, Glourcestershire; Stephen Jones, MBChB, Royal United Hospital, Avon, Somerset; Paul Mannix, MBChB, Southmead Hospital, Westbury-on-Trym, Bristol; Pamela Cairns, MBChB, St Michael’s Hospital, Bristol, Bristol; Megan Eaton, MBChB, Yeovil District Hospital, Yeovil, Somerset; Karin Schwarz, MBChB, Calderdale Royal Hospital, Halifax, West Yorkshire; David Gibson, MBChB, Pinderfields General Hospital., Dewsbury, West Yorkshire; Lawrence Miall, MBChB, Leeds Neonatal Service, Leeds, Yorkshire; David Gibson, MBChB, Pinderfields General Hospital, Wakefield, West Yorkshire; Krishnamurthy, MBChB, Walsall Manor Hospital, Walsall, West Midlands.
Appendix 2 Minimal dataset
Appendix 3 iNeo funding sources
Funding for iNeo has been provided by a Canadian Institutes of Health Research Chair in Reproductive and Child Health Services and Policy Research (APR-126340) held by PSS. The Australian and New Zealand Neonatal Network is predominantly funded by membership contributions from participating centers. The Canadian Neonatal Network is supported by a team grant from the Canadian Institutes of Health Research (CTP 87518), the Ontario Ministry of Health, and individual participating centers. The Finnish Medical Birth Register is governmentally funded and kept by the National Institute for Health and Welfare (THL). The Israel Neonatal Network very low birth weight infant database is partially funded by the Israel Center for Disease Control and the Ministry of Health. The Neonatal Research Network of Japan is partly funded by a Health Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan. SEN1500 is supported by funds from the Spanish Neonatal Society (SENeo). The Swedish Neonatal Quality Register is funded by the Swedish Government (Ministry of Health and Social Affairs) and the body of regional health care providers (County Councils). SwissNeoNet is partially funded by participating units in the form of membership fees. Tuscany Neonatal Network is funded by the Tuscany Region. The United Kingdom Neonatal Collaborative receives no core funding. MV acknowledges a research grant FIS17/0131 from the Instituto de Investigación Sanitaria Carlos III (Ministry of Science, Innovation and Universities; Kingdom of Spain) and RETICS funded by the PN 2018-2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022.
Role of the Funders/Sponsors: The funding bodies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Appendix 4 Principles of iNeo data sharing
Background
The overarching goal of iNeo collaboration is to generate new knowledge, develop quality improvement initiatives and monitor changes in outcomes and practices of neonates. In order to effectively use the dataset the following guiding principles for data sharing are developed which are based on the policy by Medical Research Council, UK.73 Results arising from the data collected and shared as a result of the iNeo collaboration are expected to meet the high standards of conducting database research including scientific quality and ethical requirements. A major impetus behind iNeo collaboration includes the provision that data arising from the iNeo collaboration will be available to the scientific community of participating networks with as few restrictions as possible so as to maximize the value of the data for research and for eventual patient and public benefit.
Currently, each network’s dataset are held by the individual networks. Within the framework of the iNeo collaboration, agreed minimal dataset for eligible infants from each network will be transferred to the iNeo Coordinating Centre after proper data transfer agreements are in place. These data will be accessed by iNeo Coordinating Centre research staff with the understanding that they are confidential to the iNeo collaboration only. Preliminary analyses will be conducted at iNeo coordinating center in Toronto.
In order to foster a true international collaboration, the data collected and housed at the iNeo Coordinating Centre will also be available to all iNeo member networks and iNeo-affiliated investigators after the principal analyses of this research proposal are completed. Specific collaborators/contributors from individual networks may take responsibility for certain analyses under the supervision of respective network director (who is a member of the iNeo Governing Board). In the initial phase, it will be preferred to send data requests to iNeo Coordinating center where analyses will be conducted and results will be sent to primary investigator(s). In the later phase of collaboration (after first year), it will be possible to either send dataset with requested variables to investigators or develop an “e-portal” where iNeo dataset could be assessed.
An individual data request will first be discussed and approved by Scientific Advisory Board (made of 2 individuals from each network). Once approved, SAB will direct the Governing Board to instruct iNeo coordinating center to conduct relevant analyses and in the later phase to release dataset to investigators. We are currently exploring the possibility of developing an “e-portal” whereby, under the supervision of their network director, researchers from all networks will be able to gain access to pre-packaged, anonymised and de-identified data subsets to address their research question. In the meantime, researchers interested in using the iNeo data will be asked to come in person to work collaboratively with statistical support available at the iNeo Coordinating Centre or to submit a request to the iNeo Coordinating Centre to have their analyses performed by members of the iNeo Coordinating Centre staff.
In all scenarios/projects involving the analyses and/or transfer of data out of the iNeo Coordinating Centre, approval of project proposals will be the responsibility of the iNeo Scientific Advisory Committee. The Scientific Advisory Committee will be formed of two members from each network and will be responsible for assessing additional projects proposed by member networks/individuals, approving requests for data transfer and analyses, evaluating the results of the primary analyses, advising member sites on knowledge translation, and taking lead roles in addressing Aims 3 and 4 of the research program. In the event that the iNeo collaboration cease to operate, the data collected will be managed according to the decision of the governing board (destruction or return the custody of dataset). At all times the iNeo Director, co-investigators and iNeo staff will ensure that data are held securely and a list of ongoing/completed analyses are made available electronically via the iNeo website (www.ineonetwork.org).
Data sharing policy
Proposals for projects requiring data sharing and transfer will be considered by the iNeo Scientific Advisory Committee. In order to safeguard the scientific integrity and validity of the data, any new studies that request data to be shared to an individual network must receive approval from the Scientific Advisory Committee who will perform a peer review of the proposed project before granting such approval. This process will evaluate and confirm that the:
- Responsibilities and rights of all parties have been agreed at the outset;
- Proposed project has a clear scientific justification, anticipated output, and timeline;
- Appropriate regulatory permissions—ethical, legal and institutional—are in place before data can be shared;
- Funds required to support data extraction and transfer are in place; and
- Proposals do not overlap with analysis that has been performed or is still being performed.
Privacy and confidentiality will be strictly maintained during any data collection and transfer according to local rules and regulations. No directly identifiable data will be collected or transmitted and in all reports only aggregate data will be presented. The iNeo Director will be responsible for coordinating applications for the use of the data generated by the iNeo collaboration. This will ensure that the iNeo Director is aware of all planned analyses and publications in order to prevent duplication and to coordinate effective dissemination of information.
The collection, collation and transfer of all data will be governed by ethical/regulatory approval from the local granting agencies of each member site and network. Approval from the Research Ethics Board at Mount Sinai Hospital, Toronto for the development, compilation, hosting and management of the iNeo dataset at the MiCare Research Centre will be obtained and maintained as up to date by the iNeo Director.
Publication of approved studies
Researchers who use the iNeo data should send the penultimate draft of any publications to the iNeo Scientific Advisory Committee to ensure correct contextual interpretation of the dataset. A standard acknowledgement that needs to be included in any publication will be provided by the iNeo coordinator. This will include but not limited to the following: “The iNeo Scientific Advisory Committee approved the use of the data included in this publication. Data were extracted from the original dataset for this study by members of the iNeo Coordinating Centre.”
Appendix 5 Publication policy
The following outlines the policy on publications based on all analysis of the iNeo dataset and any other related projects:
- No publications should precede (i) publication of iNeo protocol, and (ii) publication of the results of the primary network-level analyses, for which the iNeo Governing Board and Scientific Advisory Committee will be listed as authors;
- All publications should have “iNeo Collaboration” as final author;
- The principal primary analyses comparing outcomes between the eight networks will include the names of all participating units from all networks. This list should be referred back to in each subsequent publication;
- Publications subsequent to the publication of the primary analyses should be authored by individuals who meet the criteria for authorship as laid out by ICJME. These names should be followed by “iNeo Collaboration” as final author;
- All data requests will be handled on a first-come first-served basis. Individual investigators will have 12 months to submit their work to journals for publication. After which they may lose right to the analyses and publish the results;
- The names of the members of the iNeo Governing Board and Scientific Advisory Board should be listed in the Acknowledgements/at the end of each manuscript (subject to allowance by the journal);
- Members of the iNeo Governing Board and Scientific Advisory Board could act as authors for individual projects depending upon their contribution to the project;
- For those publications arising from projects additional to the core research proposed here, a standard acknowledgement should be included in any publication: ‘The iNeo Scientific Advisory Committee approved the use of the data included in this publication. Data were extracted from the original dataset for this study by members of the iNeo Coordinating Centre.’;
- Publications must conform to the rules of plagiarism and scientific accuracy at all times. This will be the responsibility of the authors of the publication;
- Prior to publication, all manuscripts should be submitted to the Scientific Advisory Board for their approval. The members of the Scientific Advisory Board will have 2 weeks to make a decision regarding approval and provide comments or suggestions. If no response is received, the project will be considered approved by the member of SAB;
- Disagreement or disputes regarding authorship and publication should be brought to the attention of iNeo Governing Board. In such events, the decisions of the iNeo Governing Board will be binding to all parties involved.
Acknowledgments
The authors gratefully acknowledge all investigators and data abstractors of the networks participating in the iNeo consortium for their diligent work. We thank Sarah Hutchinson, PhD from the Maternal-Infant Care Research Centre (MiCare) in Toronto, Ontario, Canada, for editorial support for this manuscript and other MiCare staff for organizational and statistical support.
Funding: This work was supported by the Canadian Institutes of Health Research [APR-126340 to P.S.S.]. The funding of individual networks is presented in Appendix 3.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
*, denotes the ANZNN Executive Committee.
References
- Kliegman RM, Rottman CJ, Behrman RE. Strategies for the prevention of low birth weight. Am J Obstet Gynecol 1990;162:1073-83. [Crossref] [PubMed]
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 1985;312:82-90. [Crossref] [PubMed]
- Moutquin J, Milot-Roy V, Irion O. Preterm birth prevention: effectiveness of current strategies. J Obstet Gynaecol Can 1996;18:571-88.
- Vermont Oxford Network. Available online: https://public.vtoxford.org/. Accessed June 21, 2019.
- Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network: 1996-1997. Pediatrics 2000;106:1070-9. [Crossref] [PubMed]
- Canadian Neonatal Network. 2019. Available online: http://www.canadianneonatalnetwork.org/portal/. Accessed June 21, 2019.
- Imperial College of London. Neonatal Data Analysis Unit. 2019. Available online: https://www.imperial.ac.uk/neonatal-data-analysis-unit/. Accessed June 14, 2019.
- Nationella Kvalitetsregister. National Quality Register for Neonatal Care (SNQ). Available online: http://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/nationalqualityregistryforneonatalcaresnq.2191.html. Accessed June 21, 2019.
- Norman M, Kallen K, Wahlstrom E, et al. The Swedish Neonatal Quality Register - contents, completeness and validity. Acta Paediatr 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Cust AE, Darlow BA, Donoghue DA, et al. Outcomes for high risk New Zealand newborn infants in 1998-1999: a population based, national study. Arch Dis Child Fetal Neonatal Ed 2003;88:F15-22. [Crossref] [PubMed]
- EXPRESS Group, Fellman V, Hellström-Westas L, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009;301:2225-33. [Crossref]
- Keirse MJ, Hanssens M, Devlieger H. Trends in preterm births in Flanders, Belgium, from 1991 to 2002. Paediatr Perinat Epidemiol 2009;23:522-32. [Crossref] [PubMed]
- Kusuda S, Fujimura M, Sakuma I, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics 2006;118:e1130-8. [Crossref] [PubMed]
- Mercier CE, Dunn MS, Ferrelli KR, et al. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology 2010;97:329-38. [Crossref] [PubMed]
- Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443-56. [Crossref] [PubMed]
- Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol 2003;27:281-7. [Crossref] [PubMed]
- Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 2002;110:143-51. [Crossref] [PubMed]
- Meadow W, Lee G, Lin K, et al. Changes in mortality for extremely low birth weight infants in the 1990s: implications for treatment decisions and resource use. Pediatrics 2004;113:1223-9. [Crossref] [PubMed]
- Shah PS, Sankaran K, Aziz K, et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol 2012;32:132-8. [Crossref] [PubMed]
- Draper ES, Zeitlin J, Fenton AC, et al. Investigating the variations in survival rates for very preterm infants in 10 European regions: the MOSAIC birth cohort. Arch Dis Child Fetal Neonatal Ed 2009;94:F158-63. [Crossref] [PubMed]
- International Neonatal Network, Scottish Neonatal Consultants, Nurses Collaborative Study Group. Risk adjusted and population based studies of the outcome for high risk infants in Scotland and Australia. Arch Dis Child Fetal Neonatal Ed 2000;82:F118-23. [Crossref] [PubMed]
- Isayama T, Lee SK, Mori R, et al. Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics 2012;130:e957-65. [Crossref] [PubMed]
- Hossain S, Shah PS, Ye XY, et al. Outcome comparison of very preterm infants cared for in the neonatal intensive care units in Australia and New Zealand and in Canada. J Paediatr Child Health 2015;51:881-8. [Crossref] [PubMed]
- Hossain S, Shah PS, Ye XY, et al. Outborns or inborns: where are the differences? A comparison study of very preterm neonatal intensive care unit infants cared for in Australia and New Zealand and in Canada. Neonatology 2016;109:76-84. [Crossref] [PubMed]
- Canadian Institutes of Health Research. CIHR Chairs in Reproductive, Child and Youth Health. 2019. Available online: http://www.cihr-irsc.gc.ca/e/46349.html. Accessed June 14, 2019.
- Shah PS, Lui K, Sjors G, et al. Neonatal outcomes of very low birth weight and very preterm neonates: an international comparison. J Pediatr 2016;177:144-52. [Crossref] [PubMed]
- Shah PS, Lee SK, Lui K, et al. The International Network for Evaluating Outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr 2014;14:110. [Crossref] [PubMed]
- Isayama T, Mirea L, Mori R, et al. Patent ductus arteriosus management and outcomes in Japan and Canada: comparison of proactive and selective approaches. Am J Perinatol 2015;32:1087-94. [Crossref] [PubMed]
- Helenius K, Sjors G, Shah PS, et al. Survival in very preterm infants: an international comparison of 10 national neonatal networks. Pediatrics 2017;140:e20171264. [Crossref] [PubMed]
- Darlow BA, Lui K, Kusuda S, et al. International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol 2017;101:1399-404. [Crossref] [PubMed]
- Lui K, Yeo KT, Darlow B, et al. Variability of nosocomial infections in very preterm neonates from 6 national neonatal networks: the International Network for Evaluation of Outcomes (iNeo) experience. San Diego, CA: Pediatric Academic Societies Annual Meeting, 2015.
- Adams M, Bassler D, Yang J, et al. Comparing necrotizing enterocolitis in extremely preterm neonates between 7 national neonatal databases: International Network for Evaluation of Outcomes (iNeo) experience. San Diego, CA: Pediatric Academic Society, 2015.
- Lui K, Modi N, Sjors G, et al. Variability in rates of major intraventricular hemorrhage (IVH) and its impact on mortality in very preterm between 8 national neonatal databases: The International Network for Evaluation of Outcomes (iNeo) experience. San Diego, CA: Presented Pediatric Academic Societies Annual Meeting, 2015.
- Norman M, Hakannson S, Kusuda S, et al. Neonatal outcome in very preterm infants with severe congenital heart defects: an international cohort study. Baltimore, MD: Pediatric Academic Societies, 2019.
- Persson M, Shah PS, Rusconi F, et al. Association of maternal diabetes with neonatal outcomes of very preterm and very low-birth-weight infants: an international cohort study. JAMA Pediatr 2018;172:867-75. [Crossref] [PubMed]
- Shah PS, Kusuda S, Hakansson S, et al. Neonatal outcomes of very preterm or very low birth weight triplets. Pediatrics 2018;142:e20181938. [Crossref] [PubMed]
- Gemmell L, Martin L, Murphy KE, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks' gestation. J Perinatol 2016;36:1067-72. [Crossref] [PubMed]
- Kelly LE, Shah PS, Hakansson S, et al. Perinatal health services organization for preterm births: a multinational comparison. J Perinatol 2017;37:762-8. [Crossref] [PubMed]
- Beltempo M, Isayama T, Vento M, et al. Respiratory management of extremely preterm infants: an international survey. Neonatology 2018;114:28-36. [Crossref] [PubMed]
- Hines D, Modi N, Lee SK, et al. Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr 2017;106:366-74. [Crossref] [PubMed]
- Darlow BA, Vento M, Beltempo M, et al. Variations in oxygen saturation targeting, and retinopathy of prematurity screening and treatment criteria in neonatal intensive care units: an international survey. Neonatology 2018;114:323-31. [Crossref] [PubMed]
- Adams M, Bassler D, Lee SK, et al. Variability in prophylactic approaches, risk factors, and rates of surgery for NEC among eight national neonatal networks. Baltimore, MD: Pediatric Academic Societies, 2018.
- Morisaki N, Helenius K, Kusuda S, et al. Variation in management of critically ill infants in preterm neonates: An international survey. San Francisco, CA: Pediatric Academic Societies, 2017.
- Morisaki N, Helenius K, Kusuda S, et al. Variations in delivery room deaths with and without active resuscitation in extremely low gestational age infants: An international survey. San Francisco, CA: Pediatric Academic Societies, 2017.
- Morisaki N, Helenius K, Kusuda S, et al. Variations in prevention and detection of neurological injury among preterm neonates <29 weeks of gestation: An international survey. San Francisco, CA: Pediatric Academic Society Annual Meeting, 2017.
- Shah PS, Lee SK, Lehtonen L, et al. Variations in physical layout, available facilities and family visiting policies for preterm neonates <29 weeks gestation: An international survey. San Francisco, CA: Pediatric Academic Society Annual Meeting, 2017.
- Shah PS, Lehtonen L, Lee SK, et al. Variations in healthcare workforce availability, allocation and developmental supportive infrastructure for preterm neonates <29 weeks gestation: An international survey. San Francisco, CA: Pediatric Academic Society Annual Meeting, 2017.
- Adams M, Shah PS, Lee SK, et al. Variations in feeding practices and probiotics usage in preterm neonates <29 weeks gestation: An international survey. San Francisco, CA: Pediatric Academic Society Annual Meeting, 2017.
- Lui K, Lee S, Kusuda S, et al. Trends in mortality and major morbidity of very preterm neonates in 10 national neonatal databases: the International Network for Evaluation of Outcomes (iNeo) experience. Toronto, ON: Pediatric Academic Society Annual Meeting, 2018.
- Costeloe K, Turner MA, Padula MA, et al. Sharing data to accelerate medicine development and improve neonatal care: data standards and harmonized definitions. J Pediatr 2018;203:437-41. [Crossref] [PubMed]
- World Health Organization. International statistical classification of diseases and related health problems 10th revision. 2010. Available online: https://icd.who.int/browse10/2010/en. Accessed June 18, 2019.
- International Health Terminology Standards Development Organization. SNOMED CT. 2019. Available online: https://www.snomed.org/snomed-ct/five-step-briefing. Accessed June 18, 2019.
- Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529-34. [Crossref] [PubMed]
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1-7. [Crossref] [PubMed]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991-9. [Crossref] [PubMed]
- Freeman J, Epstein MF, Smith NE, et al. Extra hospital stay and antibiotic usage with nosocomial coagulase-negative staphylococcal bacteremia in two neonatal intensive care unit populations. Am J Dis Child 1990;144:324-9. [PubMed]
- Shennan AT, Dunn MS, Ohlsson A, et al. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527-32. [PubMed]
- Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011;128:e348-57. [Crossref] [PubMed]
- Schmidt B, Asztalos EV, Roberts RS, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA 2003;289:1124-9. [Crossref] [PubMed]
- Battersby C, Longford N, Costeloe K, et al. Development of a gestational age-specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr 2017;171:256-63. [Crossref] [PubMed]
- Gale C, Modi N. Wheat trial development group. Neonatal randomised point-of-care trials are feasible and acceptable in the UK: results from two national surveys. Arch Dis Child Fetal Neonatal Ed 2016;101:F86-7. [Crossref] [PubMed]
- Gale C, Morris I. Neonatal Data Analysis Unit Steering Board. The UK National Neonatal Research Database: using neonatal data for research, quality improvement and more. Arch Dis Child Educ Pract Ed 2016;101:216-8. [Crossref] [PubMed]
- Profit J, Soll RF. Neonatal networks: clinical research and quality improvement. Semin Fetal Neonatal Med 2015;20:410-5. [Crossref] [PubMed]
- Lee SK, Aziz K, Singhal N, et al. Improving the quality of care for infants: a cluster randomized controlled trial. CMAJ 2009;181:469-76. [Crossref] [PubMed]
- Lee SK, Shah PS, Singhal N, et al. Association of a quality improvement program with neonatal outcomes in extremely preterm infants: a prospective cohort study. CMAJ 2014;186:E485-94. [Crossref] [PubMed]
- Shah PS, Dunn M, Aziz K, et al. Sustained quality improvement in outcomes of preterm neonates of <29 weeks' gestational age: results from the evidence-based practice for improving quality phase 3 (EPIQ-3). Can J Physiol Pharmacol 2019;97:213-21. [PubMed]



