Abstract
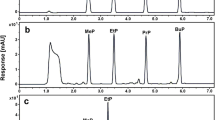
Fourteen water-miscible polar solvents were investigated for the separation from their aqueous solutions by salting-out using sodium chloride (4 mol dm-3). The following solvents showed the phase separation: acetone, acetonitrile, 1,4-dioxane, tetrahydrofuran, 1-propanol, and 2-propanol. The chemical properties of the separated organic solvents were determined by measuring Eτ(30) (=1.196×105/λ (kJ mol-1)) and DII,I (=1.196×105 (λII-1-λI-1) (kJ mol-1)) values from the spectral change of 2,6-diphenyl-4-(2,4,6-triphenylpyridinio)phenolate (DTP) and bis(1,3-propanediolato)vanadium(IV) (VO(acac)2), where λ, λIt, and λII denote the absorption maximum wavelengths (nm) of DTP and VO(acac)2. Solvent properties of acetone, acetonitrile, 1,4-dioxane, and tetrahydrofuran were dramatically altered by the salting-out. Acceptability of the phase-separated solvents increased due to the dissolution of water molecules having large acceptor numbers. The ion-pair complex of tris(1,10-phenanthroline)iron(II) chloride was easily extracted into the phase-separated acetonitrile by the salting-out. Some metal chelates of 1-(2-pyridylazo)-2-naphthol (Hpan) and 8-quinolinol (Hox), 5,10,15,20-tetraphenylporphyrin (H2tpp), and ionic species (H2ox+, ox-, and H4tpp2+) were also extracted into 1,4-dioxane. The raised donor and acceptor abilities of the phase-separated solvents allowed application to solvent extraction.
Similar content being viewed by others
References
C. E. Matkovich and G. D. Christian, Anal. Chem., 45, 1915 (1973).
B. J. Mueller and R. J. Lovett, Anal. Chem., 59, 1405 (1987).
D. C. Leggett, T. F. Jenkins, and P. H. Miyares, Anal. Chem., 62, 1355 (1990).
T. Fujinaga and Y. Nagaosa, Bull. Chem. Soc. Jpn., 53, 416 (1980).
H. Kawamoto and H. Akaiwa, Chem. Lett., 1973, 259.
W. J. Horvath and C. W. Huie, Talanta, 39, 487 (1992).
S. Igarashi and T. Yotsuyanagi, Mikrochim. Acta, 106, 37 (1992).
F. A. Long and W. F. McDevit, Chem. Rev., 51, 119 (1952).
M. Fromon and C. Treiner, J. Chem. Soc., Faraday Trans. 1, 75, 1837 (1979).
C. V. Krishnan and H. L. Friedman, J. Solution Chem., 3, 727 (1974).
C. Reichardt, “Solvents and Solvent Effects in Organic Chemistry”, VCH, Weinheim, 1988.
K. Sone and Y. Fukuda, “Inorganic Thermochromism”, Springer, New York, 1987.
R. Aveyard and R. Heselden, J. Chem, Soc., Faraday Trans. 1, 71, 312 (1975).
R. A. Pierotti, Chem. Rev., 76, 717 (1976).
V. Gutmann, “The Donor-Acceptor Approach to Molecular Interactions”, Plenum Press, New York, 1978.
V. Gutmann, “Coordination Chemistry in Non-Aqueous Solutions”, p. 22, Springer, Wien, 1968.
Y. Yamamoto, Bunseki Kagaku, 21, 418 (1972).
E. Iwamoto, J. Nishimoto, T. Yokoyama, K. Yamamoto and T. Kumamaru, J. Chem. Soc., Faraday Trans., 87, 1537 (1991).
J. Nishimoto, A. Hironaka, E. Iwamoto and T. Kumamaru, Bull. Chem. Soc. Jpn., 66, 1669 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tabata, M., Kumamoto, M. & Nishimoto, J. Chemical Properties of Water-Miscible Solvents Separated by Salting-out and Their Application to Solvent Extraction. ANAL. SCI. 10, 383–388 (1994). https://doi.org/10.2116/analsci.10.383
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.10.383