Abstract
Synopsis
Riluzole, a benzothiazole, affects neurons by 3 mechanisms: by inhibiting excitatory amino acid release, inhibiting events following stimulation of excitatory amino acid receptors and stabilising the inactivated state of voltage-dependent sodium channels. It has demonstrated neuroprotective activity in vivo and in vitro.
Results from 2 randomised double-blind placebo-controlled trials in patients with amyotrophic lateral sclerosis (ALS; motor neuron disease) have demonstrated that riluzole can extend survival and/or time to tracheostomy. After 18 months, the relative risk of death or tracheostomy with riluzole 100 mg/day was reduced by 21%. Although riluzole slowed the rate of deterioration in muscle strength in the first trial, this was not confirmed in the second, larger trial. Riluzole had no effect on any other functional or secondary variable.
Gastrointestinal effects, anorexia, asthenia, circumoral paraesthesia and dizziness were reported more frequently with riluzole than placebo. Elevated alanine aminotransferase levels were observed in 10.6 versus 3.8% of patients treated with riluzole 100 mg/day versus placebo, leading to treatment withdrawal in 3.8 versus 2.1% of patients.
In conclusion, riluzole is the first drug that has been shown to have an effect on survival in patients with ALS. Although the effect of riluzole was modest, it has allowed some insight into the pathogenesis of ALS from which future gains may be made.
Overview of Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS; motor neuron disease) is characterised by progressive muscular weakness caused by degeneration of both upper and lower motor neurons. Sensory, autonomic and oculomotor neurons are almost completely unaffected. Patients usually die of respiratory failure within a median of 3.5 years.
Both sporadic and familial types of ALS occur. The familial form of the disease has been linked to mutations in the gene encoding Cu/Zn-dependent superoxide dismutase. A number of hypotheses to explain the pathogenesis of ALS have been proposed but the precise cause of this disorder is unknown.
Pharmacodynamic Properties
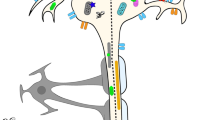
Riluzole has 3 distinct effects on neurons. At micromolar concentrations, it inhibits both the release of excitatory amino acids and N-methyl-D-aspartate (NMDA) receptor-mediated events; these effects of riluzole may occur as a result of activation of a G protein-dependent process. Riluzole also stabilises the inactivated state of voltage-dependent sodium channels at low micromolar concentrations.
Riluzole has demonstrated neuroprotective effects in vitro and in vivo. In vitro, riluzole caused partial reversal of the effects of various neurotoxins in CNS cell culture or tissue slices.
In a transgenic mouse model of ALS, riluzole significantly extended survival by 11%, although it had no effect on disease onset. Riluzole has also demonstrated neuroprotective effects in animal models of ischaemia and other neurodegenerative diseases.
Pharmacokinetic Properties
Riluzole has an average oral bioavailability of approximately 60%. Peak blood concentrations and the area under the concentration-time curve are reduced by about 45 and 20%, respectively, after a high fat meal. Steady-state plasma con-centrations of riluzole are reached within 5 days, and the drug is 96% plasma protein bound.
Riluzole is extensively metabolised (hydroxylation by cytochrome P450 1A2 and glucuronidation) in the liver. The drug has 6 major and several other minor metabolites, some of which may be pharmacologically active.
The mean elimination half-life of riluzole after multiple doses is 12 hours. After administration of [14C]-riluzole as a single dose, 90% of radioactivity is recovered in the urine and 5% in the faeces over a 7-day period.
Therapeutic Potential
The efficacy of riluzole in patients with probable or definite ALS has been investigated in 2 randomised placebo-controlled double-blind trials. The primary efficacy outcome was tracheostomy-free survival assessed by intention-to-treat analysis.
In the first trial, riluzole 100 mg/day was associated with a significantly higher rate of tracheostomy-free survival than placebo at both 12 (74 vs 58%) and 21 (49 vs 37%) months. This survival advantage was confirmed in the second, larger study (74 vs 63% at 12 months and 57 vs 50% at 18 months). At 18 months, the relative risk of tracheostomy or death with riluzole 100 mg/day was 0.79 (p = 0.076). Results obtained with riluzole 200 mg/day were similar to those with the 100 mg/day dosage.
Although riluzole slowed the rate of deterioration in muscle strength in the first trial, this was not confirmed in the second trial. Riluzole had no effect on any other functional or secondary variable.
Tolerability
Adverse events reported more commonly with riluzole than placebo were nausea, vomiting, diarrhoea, anorexia, asthenia, dizziness and circumoral paraesthesia. Treatment withdrawal was necessary in 14% of riluzole 100 mg/day recipients and 11 % of placebo recipients in one trial.
Elevated alanine aminotransferase levels (> 3 times the upper normal limit) were reported in 10.6% of riluzole 100 mg/day recipients compared with 3.8% of placebo recipients, leading to treatment withdrawal in 3.8 and 2.1% of patients, respectively. Marked neutropenia has been reported in 3 patients of approximately 4000 treated with riluzole.
Dosage and Administration
In patients with ALS, riluzole 50mg should be taken every 12 hours at least 1 hour before or 2 hours after food. Riluzole should be administered with care in patients with evidence or a history of abnormal liver function and/or renal dysfunction and also in elderly patients, in whom hepatic or renal function may be compromised by age. Serum transaminases should be monitored during riluzole therapy and frequent monitoring is recommended in those who develop elevated transaminase levels.
Riluzole (2-amino-6-trifluoromethoxybenzothiazole) is loosely termed a glutamate antagonist which has anticonvulsant, sedative and neuroprotective properties (fig. 1). Although much of the clinical research into the potential applications of this agent is at an early stage, riluzole has been tested in 2 large trials for the treatment of patients with amyotrophic lateral sclerosis (ALS; motor neuron disease). This review focuses on the use of riluzole in patients with ALS.
Similar content being viewed by others
References
Leigh PN, Ray-Chaudhuri K. Motor neuron disease. J Neurol Neurosurg Psychiatry 1994; 57(8): 886–96
Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1994; 124 Suppl.: 94–107
de Belleroche J, Orrell RW, Virgo L. Amyotrophic lateral sclerosis: recent advances in understanding disease mechanisms. J Neuropathol Exp Neurol 1996; 55(7): 747–57
de Belleroche J, Orrell R, King A. Familial amyotrophic lateral sclerosis/motor neurone disease (FALS): a review of current developments. J Med Genet 1995 Nov; 32: 841–7
Orrell RW, Habgood J, Rudge P, et al. Difficulties in distinguishing sporadic from familial amyotrophic lateral sclerosis. Ann Neurol 1996 Jun; 39: 810–2
Przedborski S, Donaldson D, Jakowec M, et al. Brain superoxide dismutase, catalase, and glutathione peroxidase activities in amyotrophic lateral sclerosis. Ann Neurol 1996; 39: 158–65
Pall HS. Motor neurone disease. Rev Clin Gerontol 1995; 5(3): 303–11
Swash M, Schwartz MS. What do we really know about amyotrophic lateral sclerosis? J Neurol Sci 1992; 113: 4–16
Appel SH, Smith RG, Engelhardt JI, et al. Evidence for autoimmunity in amyotrophic lateral sclerosis. J Neurol Sci 1993; 118: 169–74
Brown RH. Superoxide dismutase and familial amyotrophic lateral sclerosis: new insights into mechanisms and treatments. Ann Neurol 1996 Feb; 39(2): 145–6
Lipton SA, Rosenberg PA. Mechanisms of disease: Excitatory amino acids as a final common pathway for neurologic disorders. New Engl J Med 1994; 330(9): 613–22
Plaitakis A, Caroscio JT. Abnormal glutamate metabolism in amyotrophic lateral sclerosis. Ann Neurol 1987; 22: 575–9
Hugon J, Tabaraud F, Rigaud M, et al. Glutamate dehydrogenase and aspartase aminotransferase in leukocytes of patients with motor neuron disease. Neurology 1989; 39: 956–8
Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 1992 May 28; 326: 1464–8
Hubert JP, Doble A. Ibotenic acid stimulates D-[3H]aspartate release from cultured cerebellar granule cells. Neurosci Lett 1989 Jan 30; 96: 345–50
Cheramy A, Barbeito L, Godeheu G, et al. Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo. Neurosci Lett 1992 Dec 7; 147: 209–12
Martin D, Thompson MA, Nadler JV. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol 1993 Dec 21; 250: 473–6
Umemiya M, Berger AJ. Inhibition of riluzole of glycinergic postsynaptic currents in rat hypoglossal motoneurones. Br J Pharmacol 1995; 116: 3227–30
Benavides J, Camelin JC, Mitran N, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission-II: biochemical properties. Neuropharmacology 1985; 24(11): 1085–92
Hubert JP, Delumeau JC, Glowinski J, et al. Antagonism by riluzole of entry of calcium evoked by NMDA and veratridine in rat cultured granule cells: evidence for a dual mechanism of action. Br J Pharmacol 1994 Sep; 113: 261–7
Drejer J, Honore T, Schousboe A. Excitatory amino acidinduced release of 3H-GABA from cultured mouse cerebral cortex interneurons. J Neurosci 1987; 7(9): 2910–8
Girdlestone D, Dupuy A, Coston A, et al. Riluzole antagonizes excitatory amino acid-evoked firing in rat facial motoneurons in-vivo. Br J Pharmacol 1989; 97 Suppl.: 583P
Debono M-W, Le Guern J, Canton T, et al. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol 1993 Apr 28; 235: 283–9
Koek W, Colpaert FC. Selective blockade of N-methyl-D-aspartate (NMDA)-induced convulsions by NMDA antagonists and putative glycine antagonists: Relationship with phencyclidine-like behavioral effects. J Pharmacol Exp Ther 1990; 252(1): 349–57
Benoit E, Escande D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflugers Arch 1991 Dec; 419: 603–9
Hebert T, Drapeau P, Pradier L, et al. Block of the rat brain IIA sodium channel alpha subunit by the neuroprotective drug riluzole. Mol Pharmacol 1994 May; 45: 1055–60
Doble A, Hubert JP, Blanchard JC. Pertussis toxin pretreatment abolishes the inhibitory effect of riluzole and carbachol on D-[H-3]aspartate release from cultured cerebellar granule cells. Neurosci Lett 1992 Jun 22; 140: 251–4
Mantz J, Laudenbach V, Lecharny J-B, et al. Riluzole, a novel antiglutamate, blocks GABA uptake by striatal synapto-somes. Eur J Pharmacol 1994 May 12; 257: R7–8
Samuel D, Blin O, Dusticier N, et al. Effects of riluzole (2-amino-6-trifluoromethoxy benzothiazole) on striatal neuro-chemical markers in the rat, with special reference to the dopamine, choline, GABA and glutamate synaptosomal high affinity uptake systems. Fundam Clin Pharmacol 1992; 6(4-5): 177–84
Doble A, Perrier ML. Kainic acid-preferring receptors stimulate inositol phosphate formation in cultured cerebellar granule cells [abstract]. Br J Pharmacol 1989; 96: 316P
Doble A, Perrier ML. Pharmacology of excitatory amino acid receptors coupled to inositol phosphate metabolism in neonatal rat striatum. Neurochem Int 1989; 15(1): 1–8
Couratier P, Sindou P, Esclaire F, et al. Neuroprotective effects of riluzole in ALS CSF toxicity. Neuroreport 1994 Apr 14; 5: 1012–4
Estevez AG, Stutzmann J-M, Barbeito L. Protective effect of riluzole on excitatory amino acid-mediated neurotoxicity in motoneuron-enriched cultures. Eur J Pharmacol 1995 Jun 23; 280: 47–53
Malgouris C, Daniel M, Doble A. Neuroprotective effects of riluzole on N-methyl-D-aspartate- or veratridine-induced neurotoxicity in rat hippocampal slices. Neurosci Lett 1994 Aug 15; 177: 95–9
Rothstein JD, Kuncl RW. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem 1995 Aug; 65: 643–51
Gurney ME, Cutting FB, Zhai P, et al. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol 1996; 39: 147–57
Malgouris C, Bardot F, Daniel M, et al. Riluzole, a novel antiglutamate, prevents memory loss and hippocampal neuronal damage in ischemic gerbils. J Neurosci 1989 Nov; 9: 3720–7
Pratt J, Rataud J, Bardot F, et al. Neuroprotective actions of riluzole in rodent models of global and focal cerebral ischaemia. Neurosci Lett 1992 Jun 22; 140: 225–30
Wahl F, Allix M, Plotkine M, et al. Effect of riluzole on focal cerebral ischemia in rats. Eur J Pharmacol 1993 Jan 12; 230: 209–14
Stutzmann J-M, Pratt J, Boraud T, et al. The effect of riluzole on post-traumatic spinal cord injury in the rat. Neuroreport 1996 Jul; 7: 387–92
Benazzouz A, Boraud T, Dubedat P, et al. Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study. Eur J Pharmacol 1995 Sep 25; 284: 299–307
Boireau A, Dubedat P, Bordier F, et al. Riluzole and experimental parkinsonism: antagonism of MPTP-induced decrease in central dopamine levels in mice. Neuroreport 1994 Dec 20; 5: 2657–60
Mary V, Wahl F, Stutzmann JM. Effect of riluzole on quinolinate-induced neuronal damage in rats: comparison with blockers of glutamatergic neurotransmission. Neurosci Lett 1995; 201: 92–6
Mizoule J, Meldrum B, Mazadier M, et al. 2-Amino-6- trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission-I: anticonvulsant properties. Neuropharmacology 1985 Aug; 24: 767–73
Stutzmann J-M, Bohme GA, Gandolfo G, et al. Riluzole prevents hyperexcitability produced by the mast cell degranulating peptide and dendrotoxin I in the rat. Eur J Pharmacol 1991 Feb 1; 193: 223–9
Romettino S, Lazdunski M, Gottesmann C. Anticonvulsant and sleep-waking influences of riluzole in a rat model of absence epilepsy. Eur J Pharmacol 1991 Jul 9; 199: 371–3
Serrano A, D’Angio M, Scatton B. NMDA antagonists block restraint-induced increase in extracellular DOPAC in rat nucleus accumbens. Eur J Pharmacol 1989 Mar 14; 162: 157–66
Stutzmann J-M, Cintrat P, Laduron PM, et al. Riluzole antagonizes the anxiogenic properties of the beta-carboline FG7142 in rats. Psychopharmacology 1989; 99(4): 515–9
Mantz J, Chéramy A, Thierry A-M, et al. Anesthetic properties of riluzole (54274 RP), a new inhibitor of glutamate neurotransmission. Anesthesiology 1992 May; 76: 844–8
Saletu B, Grünberger J, Anderer P, et al. Effects of the novel neuroprotective agent, riluzole, on human brain function and behavior: I. Double-blind, placebo-controlled EEG mapping and psychometric studies under normoxia. Methods Find Exp Clin Pharmacol 1996; 18(1): 55–66
Rhône-Poulenc Rorer. Riluzole prescribing information. Collegeville, PA, USA, 1996
Bensimon G, Lacomblez L, Meininger V, et al. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 1994 Mar 3; 330: 585–91
Lacomblez L, Bensimon G, Leigh PN, et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet 1996 May 25; 347: 1425–31
Rowland LP. Riluzole for the treatment of amyotrophic lateral sclerosis — too soon to tell? N Engl J Med 1994 Mar 3; 330: 636–7
MacRae KD. Riluzole in amyotrophic lateral sclerosis [letter]. N Engl J Med 1994 Jul 28; 331: 272–3
Murphy JR. Riluzole in amyotrophic lateral sclerosis [letter]. N Engl J Med 1994 Jul 28; 331: 273
Askmark H, Aquilonius S-M. Amyotrophic lateral sclerosis: practical treatment recommendations. CNS Drugs 1994 Aug; 2: 102–9
Orrell RW, Lane RJM, Guiloff RJ. Recent developments in the drug treatment of motor neurone disease: nothing works yet; many potential treatments remain uninvestigated. BMJ 1994 Jul 16; 309: 140–1
Plaitakis A, Fesdjian CO, Shashidharan P. Glutamate antagonists in amyotrophic lateral sclerosis: a review of their therapeutic potential. CNS Drugs 1996 Jun; 5(6): 437–56
Borasio GD, de Jong JMBV, Emile J, et al. Insulin-like growth factor I in the treatment of amyotrophic lateral sclerosis: results of the European multicenter, double-blind, placebo-controlled trial [abstract no. 138]. Neurology 1996; 243(6) Suppl. 2: S26
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: H. Askmark, Department of Neurology, Länssjukhuset, Halmstad, Sweden; O. Blin, Centre de Pharmacologie Clinique et d/’Evaluations Therapeutiques, Hopital de la Timone, Marseilles, France; P. Drapeau, Montreal General Hospital Research Institute, Montreal, Quebec, Canada; P.N. Leigh, Department of Neurology, Institute of Psychiatry, London, England; V. Meininger, Service de Neurologie, Division Mazarin, Hôpital de la Salpêtrière, Paris, France; R.G. Miller, Department of Neurology, California Pacific Medical Center, San Francisco, California, USA; K.W. Muir, Department of Neurology, Institute of Neurological Sciences, Southern General Hospital, Glasgow, Scotland; J.V. Nadler, Department of Pharmacology, Duke University Medical Center, Durham, North Carolina, USA; R.W. Orrell, Neuromuscular Unit, Regional Centre and University Department of Clinical Neuroscience, Charing Cross Hospital, London, England; A. Plaitakis, Mount Sinai School of Medicine, New York, New York, USA; M. Umemiya, Department of Neurophysiology, Tohoku University School of Medicine, Sendai, Japan; M. Yasui, Division of Neurological Diseases, Wakayama Medical College, Wakayama, Japan.
Rights and permissions
About this article
Cite this article
Bryson, H.M., Fulton, B. & Benfield, P. Riluzole. Drugs 52, 549–563 (1996). https://doi.org/10.2165/00003495-199652040-00010
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199652040-00010