Abstract
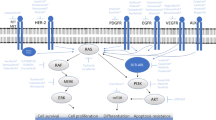
Abnormal cell signal transduction arising from protein tyrosine kinases has been implicated in the initiation and progression of a variety of human cancers. Over the past 2 decades pharmaceutical and university laboratories have been involved in a tremendous effort to develop compounds that can selectively modulate these abnormal signalling pathways. Targeting receptor tyrosine kinases, especially the epidermal growth factor receptor subfamily, has been at the forefront of this effort as a result of strong clinical data correlating over-expression of these receptors with more aggressive cancers.
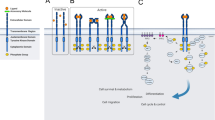
There are a variety of strategies under development for inhibiting the kinase activity of these receptors, targeting both the extracellular and intracellular domains. Antibody-based approaches, immunotoxins and ligand-binding cytotoxic agents use the extracellular domain for targeted tumour therapy. Small molecule inhibitors target the intracellular catalytic region by interfering with ATP binding, while nonphosphorylatable peptides are aimed at the intracellular substrate binding region. Compounds that inhibit subsequent downstream signals from the receptor by interrupting intracellular protein recognition sequences are also being investigated.
In the past 5 years enormous progress has been made in developing tyrosine kinase inhibitor compounds with sufficient potency, bioavailability and selectivity against this subfamily of receptor tyrosine kinases. The anti-HER2 monoclonal antibody, trastuzumab, for patients with metastatic breast cancer is the first of these inhibitor compounds to gain FDA approval. However, preclinical and clinical trials are ongoing with a variety of other monoclonal antibodies, immu-notoxins, and small molecule quinazoline and pyrimidine-based inhibitors. Although their cytotoxic and cytostatic potential has been proven, they are not likely to replace standard chemotherapy regimens as single-agent, first-line therapeutics. Instead, their promising additive and synergistic antitumour effects in combination with standard chemotherapeutics suggest that these novel agents will find their greatest utility and efficacy in conjunction with existing anticancer agents.



Similar content being viewed by others
References
Sutherland EW, Wosilait WD. Inactivation and activation of liver phosphorylase. Nature 1955; 175: 169–70
Fischer EH, Krebs EG. Conversion of phosphorylase b to phosphorylase a in muscle extracts. J Biol Chem 1955; 216: 121–32
Radke K, Gilmore T, Martin GS. Transformation by Rous sarcoma virus: a cellular substrate for transformation specific protein phosphorylation contains phosphotyrosine. Cell 1980; 21: 821–3
Hunter T. Cooperation between oncogenes. Cell 1991; 64: 249–70
Hunter T. 1001 protein kinases redux — towards 2000. Semin Cell Biol 1994; 5: 367–76
Johnson LN, Lowe ED, Martin EM, et al. The structural basis for substrate recognition and control by protein kinases. FEBS Lett 1998; 430: 1–11
Knighton DR, Zheng JH, Ten Eyck LF, et al. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 1991; 253: 407–13
Mohammadi M, McMahon G, Sun L, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 1997; 276: 955–60
Hubbard SR, Wei L, Ellis L, et al. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature 1994; 372: 746–54
Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domain. Science 1988; 241: 48–82
Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 1995; 9(8): 576–96
The Protein Kinase Resource database. Available from: URL: http://www.sdsc.edu/kinases/ [Accessed 2000 Jan 5]
Hanks S, Quinn AM. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol 1991; 200: 38–62
McTigue MA, Wickersham JA, Pinko C, et al. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure 1999; 7(3): 319–30
Meng W, Sawasdikosol S, Burakoff SJ, et al. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 1999; 398: 84–90
Lamers MB, Antson AA, Hubbard RE, et al. Structure of the protein tyrosine kinase domain of the C-terminal Src kinase (CSK) in complex with staurosporine. J Mol Biol 1999; 285: 713–25
Himanen JP, Henkemeyer M, Nikolov DB. Crystal structure of the ligand-binding domain of the receptor tyrosine kinase EphB2. Nature 1998; 396: 486–91
Mohammadi M, Froum S, Hamby JM, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J 1998; 17(20): 5896–904
Garrett TP, McKern NM, Lou M, et al. Crystal structure of the first three domains of the type-1 insulin-like growth factor receptor. Nature 1998; 394: 395–9
Chiao PJ, Bischoff FZ, Strong LC, et al. The current status of oncogenes and cancer: experimental approaches for analyzing oncogenetic events in human cancer. Cancer Metastasis Rev 1990; 9: 63–80
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER2/neu oncogene. Science 1987; 235: 177–82
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER2 proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–12
Macias A, Azavedo E, Hagerstrom T, et al. Prognostic significance of the receptor for epidermal growth factor receptor in human mammary cancers. Anticancer Res 1987; 7(3): 459–64
Weber TK, Steele G, Summerhayes LC. Differential pp60-src activity in well and poorly differentiated human carcinomas and cell lines. J Clin Invest 1992; 90: 815–21
Irby RB, Mao W, Coppola D, et al. Activating SRC mutation in a subset of advanced human colon cancers. Nature Gen 1999; 21(2): 187–90
Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science 1995; 267: 1782–8
Klohs WD, Fry DW, Kraker AJ. Inhibitors of tyrosine kinase. Curr Opin Oncol 1997; 9: 562–8
Lawrence DS, Niu J. Protein kinase inhibitors: the tyrosine specific protein kinases. Pharmacol Ther 1998; 77(2): 81–114
Shawver L. Tyrosine kinase inhibitors: from the emergence of targets to their clinical development. In: Perry MC, editor. American Society of Clinical Oncology Educational Book 35. Alexandria: ASCO Spring, 1999: 29–47
Showalter HDH, Kraker AJ. Small molecule inhibitors of the platelet-derived growth factor receptor, the fibroblast growth factor receptor, and the Src family tyrosine kinases. Pharmacol Ther 1997; 76(1–3): 55–71
Drebin JA, Link VC, Stern DF, et al. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 1985; 41: 695–706
Hudziak RM, Lewis GD, Winget M, et al. p185 HER2 monoclonal antibody has antiproliferative effects in vivo and sensitizes cells to tumor necrosis factor. Mol Cell Biol 1989; 9: 1165–72
Hancock MC, Langton BC, Chan T, et al. A monoclonal antibody against the c-erbB2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res 1991; 51: 4575–80
Baselga J, Tripathy D, Mendelsohn J, et al. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the anti-tumor activity of paclitaxel and doxorubicin against HER2/ neu overexpressing human breast cancer xenografts. Cancer Res 1996; 58: 2825–31
Benz C, Tripathy D. ErbB2 overexpression in breast cancer: biology and clinical translation. J Womens Cancer 2000; 2: 33–40
Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor enhanced chemosensitivity using recombinant humanized anti-p185 HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998; 16: 2659–71
Slamon D, Leyland-Jones B, Shak S, et al. Addition of Herceptin (humanized anti-HER2 antibody) to first line chemotherapy for HER2 overexpressing metastatic breast cancer (HER2+/MBC) markedly increases anticancer activity: a randomized multinational phase III trial. Proc Am Soc Clin Oncol 1998; 17: 377
Scott GK, Robles R, Park JW, et al. A truncated intracellular HER2/neu receptor produced by alternate RNA processing affects growth of human carcinoma cells. Mol Cell Biol 1993; 13: 2247–57
Park J, Colbern G, Nuijens A, et al. Increased levels of circulating HER2 ECD in response to anti-HER2 antibody therapy. Breast Cancer Res Treat 1997; 46: 267
Doherty J, Ramsey E, Keenan E, et al. N-terminally truncated HER2/neu kinase is related to shedding of the extracellular domain and is associated with lymph node metastasis in breast cancer. Breast Cancer Res Treat 1998; 50: 306
Gebhardt F, Zanker K, Brandt B. Differential expression of alternatively spliced c-erbB2 mRNA in primary tumors, lymph node metastases and bone marrow micrometastases from breast cancer patients. Biochem Biophys Res Comm 1998; 247: 319–23
Hoffmann T, Hafner D, Ballo H, et al. Antitumor activity of anti-epidermal growth factor receptor monoclonal antibodies and cisplatin in ten head and neck squamous cell carcinoma lines. Anticancer Res 1997; 17: 4419–25
Modjtahedi H, Affleck K, Stubberfield C, et al. EGFR blockade by tyrosine kinase inhibitor or monoclonal antibody inhibits growth, directs terminal differentiation and induces apoptosis in the human squamous cell carcinoma HN5. Int J Oncol 1998; 13(2): 335–42
Yang XD, Jia XC, Corvalan JR, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res 1999; 59: 1236–43
Mendelsohn J. Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin Cancer Res 1997; 3(12): 2703–7
Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1995; 1(11): 1311–8
Wu X, Rubin M, Fan Z, et al. Involvement of p27KIPl in G1 arrest mediated by anti-EGFR monoclonal antibodies. Oncogene 1996; 12(7): 1397–403
Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res 1999; 5(2): 257–65
Ciardello F, Bianco R, Damiano V, et al. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res 1999; 5(4): 909–16
Gullick WJ. Inhibitors of growth factor receptors. In: Carney WA, Sikora K, editors. Genes and cancer. Chichester: Wiley, 1990: 263–73
Tang CK, Lippman ME. EGF family receptors and their ligands in human cancer. Horm Signal 1998; 1: 113–65
Negri DR, Tosi E, Valota O, et al. In vitro and in vivo stability and anti-tumour efficacy of an anti-EGFR/anti-CD3 F(ab)2 bispecific monoclonal antibody. Br J Cancer 1995; 72(4): 928–33
Curnow RT. Clinical experience with CD64-directed immunotherapy: an overview. Cancer Immunol 1997; 45: 210–5
Pfosser A, Brandi M, Salih H, et al. Role of target antigen in bispecific-antibody-mediated killing of human glioblastoma cells: a preclinical study. Int J Cancer 1999; 80(4): 612–6
Valone FH, Kaufman PA, Guyre PM, et al. Phase Il/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER2/neu. J Clin Invest 1995; 13: 2281–92
Goldstein J, Graziano RF, Sundarapandiyan K. Cytotoxic and cytostatic properties of an anti-human Fc gamma R1 × epidermal growth factor receptor bispecific fusion protein. J Clin Immunol 1997; 158: 872–9
Keler T, Graziano RF, Mandai A, et al. Bispecific antibody-dependent cellular cytotoxicity of HER2/neu overexpressing tumor cells by Fc gamma receptor type 1 expressing effector cells. Cancer Res 1997; 57(18): 4008–14
Manzke O, Tesch H, Diehl V, et al. Single-step purification of bispecific monoclonal antibodies for immunotherapeutic use by hydrophobic interaction chromatography. J Immunol Methods 1997; 208: 65–73
Graus-Porta D, Beerli RR, Hynes NE. Single chain antibody-mediated intracellular retention of ErbB2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol 1995; 15(3): 1182–91
Raag R, Whitlow M. Single-chain Fvs. FASEB J 1995; 9(1): 73–80
Peterson NC, Greene MI. Bacterial expression and characterization of recombinant biologically active anti-tyrosine kinase receptor antibody forms. DNA Cell Biol 1998; 17: 1031–40
Beerli RR, Wels W, Hynes NE. Inhibition of signaling from Type 1 receptor tyrosine kinases via intracellular expression of single-chain antibodies. Breast Cancer Res Treat 1996; 38: 11–7
Jannot CB, Beerli RR, Mason S, et al. Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cells. Oncogene 1996; 13(2): 275–82
Schmidt M, Reiser P, Hills D, et al. Expression of an oncogenic mutant EGF receptor markedly increases the sensitivity of cells to an EGF-receptor-specific antibody toxin. Int J Cancer 1998; 75(6): 878–84
Schmidt M, Maurer-Gebhard M, Groner B, et al. Suppression of metastatic formation by a recombinant single chain antibody-toxin targeted to the full-length and oncogenic variant EGF receptors. Oncogene 1999; 18(9): 1711–21
Maurer-Gebhard M, Schmidt M, Azemar M, et al. Systemic treatment with a recombinant erbB2 receptor-specific tumor toxin efficiently reduces pulmonary metastases in mice injected with genetically modified carcinoma cells. Cancer Res 1998; 58(12): 2661–6
Rosenblum MG, Shawver LK, Marks JW, et al. Recombinant immunotoxins directed against the c-erbB2/HER2/neu oncogene product: in vitro cytotoxicity, pharmacokinetics, and in-vivo efficacy studies in xenograft models. Clin Cancer Res 1999; 5(4): 865–74
Fischer PH, Bird RE, Kasprzyk PG, et al. In vitro and in vivo activity of a recombinant toxin OLX-209, which targets the erbB2 oncoprotein. Adv Enzymol 1994; 34: 119–28
Kasprzyk PG, Sullivan TL, Hunt JD, et al. Activity of anti-erbB2 recombinant toxin OLX-209 on lung cancer cell lines in the absence of erbB2 gene amplification. Clin Cancer Res 1996; 2(1): 75–80
King CR, Kasprzyk PG, Fischer PH, et al. Preclinical testing of an anti-erbB2 recombinant toxin. Breast Cancer Res Treat 1996; 38(1): 19–25
Schmidt M, Wels W. Targeted inhibition of tumor cell growth by a bispecific single chain toxin containing an antibody domain and TGF alpha. Br J Cancer 1996; 74(6): 853–62
Schmidt M, Hynes NE, Groner B, et al. A bivalent single chain antibody-toxin specific for erbB2 and the EGF receptor. Int J Cancer 1996; 65(4): 538–46
Yang D, Kuan C-T, Payne J, et al. Recombinant heregulin-pseudomonas exotoxin fusion proteins: interactions with the heregulin receptors and antitumor activity in vivo. Clin Cancer Res 1998; 4: 993–1004
Siegall CB, Fitzgerald DJ, Pastan I. Selective killing of tumor cells using EGF or TGF alpha-pseudomonas exotoxin chimeric molecules. Semin Cancer Biol 1990; 1(5): 345–50
Chandler LA, Sosnowski BA, McDonald JR, et al. Targeting tumor cells via EGF receptors: selective toxicity of an HBEGF-toxin fusion protein. Int J Cancer 1998; 78(1): 106–11
Yoon JM, Han SH, Kown OB, et al. Cloning and cytotoxicity of fusion proteins of EGF and angiogenin. Life Sci 1999; 64(16): 1435–45
Psarras K, Ueda M, Yamamura T, et al. Human pancreatic Rnasel-human epidermal growth factor fusion: an entirely human immunotoxin analogue with cytotoxic properties against squamous cell carcinomas. Protein Eng 1998; 11(12): 1285–92
Fitzgerald D. Why toxins! Semin Cancer Biol 1996; 7(2): 87–95
Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 1987; 262(12): 5592–5
Uckun FM, Narla RK, Zeren T, et al. In vivo toxicity, pharmacokinetics, and anticancer activity of genistein linked to recombinant human epidermal growth factor. Clin Cancer Res 1998; 4: 1125–34
Uckun FM, Narla RK, Jun X, et al. Cytotoxic activity of epidermal growth factor-genistein against breast cancer cells. Clin Cancer Res 1998; 4: 901–12
Betsholtz C, Johnsson A, Heldin CH, et al. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A 1986; 83(17): 6440–4
Stein CA. Suramin: a novel neoplastic agent with multiple potential mechanisms of action. Cancer Res 1993; 53: 2239–48
Lozano RM, Jimenez MA, Santoro J, et al. Solution structure of acidic fibroblast growth factor bound to 1,3,6-napthalenetrisulfonate: a minimal model for the anti-tumoral action of suramins and suradistas. J Mol Biol 1998; 281: 899–915
Shin R, Naomoto Y, Kamikawa Y, et al. Effect of surmamin on human esophageal cancer cells in vitro and in vivo. Scand J Gastroenterol 1997; 32(8): 824–8
Fujiuchi S, Ohsaki Y, Kikuchi K. Suramin inhibits the growth of non-small-cell lung cancer cells that express the epidermal growth factor receptor. Oncology 1997; 54(2): 134–40
Umezawa H, Imoto M, Sawa T, et al. Studies on a new epidermal growth factor-receptor kinase inhibitor, erbstatin, produced by MH435-hF3. J Antibiotics 1986; 39(1): 170–3
Ghosh S, Zheng Y, Jun X, et al. Alpha-cyano-beta-hydroxybeta-methyl-N-[4-(trifluoromethoxy)phenyl] propenamide: an inhibitor of the epidermal growth factor receptor tyrosine kinase with potent cytotoxic activity against breast cancer cells. Clin Cancer Res 1998; 4(11): 2657–68
Sudbeck EA, Liu XP, Narla RK, et al. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin. Cancer Res. 1999; 5(6): 1569–82
Mahajan S, Ghosh S, Sudbeck EA, et al. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton’s tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2,5-dibromophenyl)propenamide]. J Biol Chem 1999; 274: 9587–99
Fry DW, Kraker AT, McMichael A, et al. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 1994; 265: 1093–5
Traxler P, Furet P, Mett H, et al. 4-(phenylamino)pyrrolopyrimidines: potent and selective ATP site directed inhibitors of the EGF-receptor protein tyrosine kinase. J Med Chem 1996; 39(12): 2285–92
Rewcastle GW, Palmer BD, Thompson AM, et al. Tyrosine kinase inhibitors: 10. Isomeric 4-[(3-bromophenyl)amino]-pyrido-d-pyrimidines are potent ATP binding site inhibitors of the tyrosine kinase function of the epidermal growth factor receptor. J Med Chem 1996; 39: 1823–35
Woodburn JR, Barker AJ, Gibson KH, et al. ZD1839, an epidermal growth factor tyrosine kinase inhibitor selected for clinical development [abstract 4251]. Proceedings of the 88th Annual Meeting of the American Association for Cancer Research: 1997 Apr 12–16; San Diego (CA), 633
Iwata K, Miller PE, Barbacci EG, et al. CP-358,774: a selective EGFR kinase inhibitor with potent antiproliferative activity against head and neck tumor cells [abstract 4248]. Proceedings of the 88th Annual Meeting of the American Association for Cancer Research: 1997 Apr 12–16; San Diego (CA), 633
Fry DW, Bridges AJ, Denny WA, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci U S A 1998; 95: 12022–7
Traxler P, Bold G, Frei J, et al. Use of a pharmacore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenyl-amino)pyrazolo[3,4-d]pyrimidines. J Med Chem 1997; 40(22): 3601–16
Elliot WL, Fry DW, Vincent PW, et al. In vitro and in vivo activity of4-anilinopyridopyrimidine EGF family specific tyrosine kinase inhibitors [abstract]. Proceedings of the 88th Annual Meeting of the American Association for Cancer Research: 1997 Apr 12–16; San Diego (CA), 470
Denny WA, Palmer BD, Rewcastle GW, et al. Pyrido[d]pyrimidine inhibitors of the tyrosine kinase activity of the EGF receptor: a binding model and structure-activity relationships for soluble analogues [abstract]. Proceedings of the 88th Annual Meeting of the American Association for Cancer Research: 1997 Apr 12–16; San Diego (CA), 633
Rewcastle GW, Murray DK, Elliot WL, et al. Tyrosine kinase inhibitors. 14. Structure-activity relationships for methylamino-substituted derivatives of 4-[(3-bromophenyl)amino]-6-(methylamino)-pyrido[3,4]pyrimidine (PD158780), a potent and specific inhibitor of the tyrosine kinase activity of receptors for the EGF family of growth factors. J Med Chem 1998; 41(5): 742–51
Thompson AM, Fry DW, Kraker A, et al. Tyrosine kinase inhibitors: 2. Synthesis of 2,2′-dithio-bis(1H-indole-3-alkanamides) and investigation of their inhibitory activity against the epidermal growth factor receptor and pp60src protein tyrosine kinases. J Med Chem 1994; 37: 598–609
Buchdunger E, Trinks U, Mett H, et al. 4,5-dianilinophthalimide: a protein tyrosine kinase inhibitor with selectivity for the epidermal growth factor receptor signal transduction pathway and potent in vivo antitumor activity. Proc Natl Acad Sci U S A 1994; 91: 2234–8
Dinney CP, Parker C, Dong Z, et al. Therapy of human transitional cell carcinoma of the bladder by oral administration of the epidermal growth factor protein kinase inhibitor 4,5-dianilinophthalimide. Clin Cancer Res 1997; 3(2): 161–8
Zhang L, Lau YK, Xi L, et al. Tyrosine kinase inhibitors, emodin and its derivatives repress HER2/neu-induced cellular transformation and metastasis-associated properties. Oncogene 1998; 16(22): 2855–63
Copp BR, Fairchild CR, Cornell L, et al. Naamidine A is an antagonist of the epidermal growth factor receptor and an in vivo active antitumor agent. J Med Chem 1998; 41(20): 3909–11
Hinterding K, Knebel A, Herrlich P, et al. Synthesis and biological evaluation of aeroplysinin analogues: a new class of receptor tyrosine kinase inhibitors. Bioorg Med Chem 1998; 6(8): 1153–62
Fry DW, McMichael A, Singh J. Design of a potent peptide inhibitor of the epidermal growth factor receptor tyrosine kinase utilizing sequences based on the natural phosphorylation sites of phosphholipase C-γ-1. Peptides 1994; 15: 951–7
Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analogue. EMBO J 1997; 16(18): 5572–81
Fong TA, Shawver LK, Sun L, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (flk/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 1999; 59(1): 99–106
Uckun FM, Evans WE, Forsyth CJ, et al. Biotherapy of B-cell precursor leukemia by targeting genistein to CD19-associated tyrosine kinases. Science 1995; 886–91
Ek O, Yanishevski Y, Zeren T, et al. In vivo toxicity and pharmacokinetic features of B43(anti-CD19)-genistein immunoconjugate. Leuk Lymphoma 1998; 30: 389–94
Beren M, Cao X, Estrov Z, et al. Selective inhibition of cell proliferation and BCR-ABL phosphorylation in acute lymphoblastic leukemia cells expressing Mw 190,000BCR-ABL protein by a tyrosine kinase inhibitor (CGP-57148). Clin Cancer Res 1998; 4: 1661–72
le Courre P, Mologni L, Cleris L, et al. In vivo eradication of human BCR/ABL-positive leukemia cells with an ABL kinase inhibitor. J Natl Cancer Inst 1999; 91(2): 163–8
Bhatia R, Munthe HA, Verfaillie CM. Tyrphostin AG957, a tyrosine kinase inhibitor with anti-BCR/ABL tyrosine kinase activity restores betal integrin-mediated adhesion and inhibitory signaling in chronic myelogenous leukemia hematopoietic progenitors. Leukemia 1998; 12(11): 1708–17
Guo XY, Cuillerot JM, Wang T, et al. Peptide containing the BCR oligomerization domain (AA 1-160) reverses the transformed phenotype of p210bcr-abl positive 32D myeloid leukemia cells. Oncogene 1998; 825–33
Clackson T. Redesigning small molecule-protein interfaces. Curr Opin Struct Biol 1998; 8(4): 451–8
Levitzki A. Src as a target for anticancer drugs. Anticancer Drug Des 1996; 11: 175–82
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Noonberg, S.B., Benz, C.C. Tyrosine Kinase Inhibitors Targeted to the Epidermal Growth Factor Receptor Subfamily. Drugs 59, 753–767 (2000). https://doi.org/10.2165/00003495-200059040-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200059040-00003