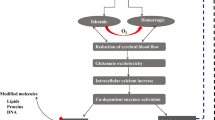
Abstract
It is now clear that reactive oxygen species (ROS) can act as signalling molecules in the cerebral circulation under both physiological and pathological conditions. Some major products of superoxide (O2•−) metabolism, such as hydrogen peroxide (H2O2) and hydroxyl radical (OH•), appear to be particularly good cerebral vasodilators and may, surprisingly, represent important molecules for increasing local cerebral blood flow.
A major determinant of overall ROS levels in the cerebral circulation is the rate of generation of the parent molecule, O2•−. Although the major enzymatic source of O2•− in cerebral arteries is yet to be conclusively established, the two most likely candidates are cyclo-oxygenase and nicotinamide adenine dinucleotide phosphate (reduced form) [NADPH] oxidase. The activity of endogenous superoxide dismutases (SODs) play a vital role in determining levels and effects of all individual ROS derived from metabolism of O2•−.
The term ‘oxidative stress’ may be an over-simplification that hides the complexity and diversity of the ROS family in cerebrovascular health and disease. Although a generalised increase in ROS levels seems to occur during several vascular disease states, the consequences of this for cerebrovascular function are still unclear.
Because enhanced breakdown of O2•− by SOD will increase the generation of the powerful cerebral vasodilator H2O2, this latter molecule could conceivably act as a compensatory vasodilator mechanism in the cerebral circulation under conditions of elevated O2•− production.
Some recent clinical data support the concept of a protective role for cerebrovascular NADPH oxidase activity. Although it is quite speculative at present, if NADPH oxidase were to emerge as a major source of beneficial vasodilator ROS in the cerebral circulation, this may represent a significant dilemma for treatment of ischaemic cerebrovascular conditions, as excessive NADPH oxidase activity is associated with the progression of several systemic vascular disease states, including hypertension and atherosclerosis.
Despite data suggesting that antioxidant vitamins can have beneficial effects on vascular function and that their plasma levels are inversely correlated with risk of cardiovascular disease and stroke, the results of several recent large-scale clinical trials of antioxidant supplementation have been disappointing.
Future work must establish whether or not increased ROS generation is necessarily detrimental to cerebral vascular function, as has been generally assumed, or whether localised increases in ROS in the vicinity of the arterial wall could be beneficial in disease states for the maintenance of cerebral blood flow.


Similar content being viewed by others
References
Rosenblum WI. Effects of free radical generation on mouse pial arterioles: probable role of hydroxyl radicals. Am J Physiol1983; 245(1): H139–42
Wei EP, Christman CW, Kontos HA, et al. Effects of oxygen radicals on cerebral arterioles. Am J Physiol 1985; 248 (2 Pt 2): H157–62
Leffler CW, Busija DW, Armstead WM, et al. H2O2 effects on cerebral prostanoids and pial arteriolar diameter in piglets. Am J Physiol1990; 258 (5 Pt 2): H1382–7
Wei EP, Kontos HA. H2O2 and endothelium-dependent cerebral arteriolar dilation: implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension 1990; 16(2): 162–9
Iida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke 2000; 31(9): 2224–30
Sobey CG, Heistad DD, Faraci FM. Potassium channels mediate dilatation of cerebral arterioles in response to arachidonate. Am J Physiol 1998; 275 (5 Pt 2): H1606–12
Sobey CG, Heistad DD, Faraci FM. Mechanisms of bradykinin-induced cerebral vasodilatation in rats: evidence that reactive oxygen species activate K+ channels. Stroke 1997; 28(11): 2290–4
Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol 1996; 271(3 Pt 2): H1262–6
Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 2000; 20(6): 1430–42
Skinner KA, White CR, Patel R, et al. Nitrosation of uric acid by peroxynitrite: formation of a vasoactive nitric oxide donor. J Biol Chem 1998; 273(38): 24491–7
Kontos HA, Wei EP, Povlishock JT, et al. Oxygen radicals mediate the cerebral arteriolar dilation from arachidonate and bradykinin in cats. Circ Res 1984; 55(3): 295–303
Rosenblum WI. Hydroxyl radical mediates the endothelium-dependent relaxation produced by bradykinin in mouse cerebral arterioles. Circ Res 1987; 61(4): 601–3
Ellis EF, Police RJ, Yancey L, et al. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol 1990; 259 (4 Pt 2): H1171–7
Yang ST, Mayhan WG, Faraci FM, et al. Mechanisms of impaired endothelium-dependent cerebral vasodilatation in response to bradykinin in hypertensive rats. Stroke 1991; 22(9): 1177–82
Matoba T, Shimokawa H, Nakashima M, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 2000; 106(12): 1521–30
Matoba T, Shimokawa H, Kubota H, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 2002; 290(3): 909–13
Yada T, Shimokawa H, Hiramatsu O, et al. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 2003; 107(7): 1040–5
Matoba T, Shimokawa H, Morikawa K, et al. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 2003; 23(7): 1224–30
Morikawa K, Shimokawa H, Matoba T, et al. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyper-polarization. J Clin Invest 2003; 112(12): 1871–9
Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol 2001; 281(4): H1697–703
Griendling KK, Sorescu D, Ushio-Fukai M. NAD (P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000; 86(5): 494–501
Landino LM, Crews BC, Timmons MD, et al. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A 1996; 93(26): 15069–74
Babior BM. NADPH oxidase: an update. Blood 1999; 93(5): 1464–76
Bengtsson SHM, Gulluyan LM, Dusting GJ, et al. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol 2003; 30: 849–54
Lassegue B, Sorescu D, Szocs K, et al. Novel gp91 (phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 2001; 88(9): 888–94
Sorescu D, Weiss D, Lassegue B, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002; 105(12): 1429–35
Ago T, Kitazono T, Ooboshi H, et al. Nox4 as the major catalytic component of an endothelial NAD (P)H oxidase. Circulation 2004; 109: 227–33
De Keulenaer GW, Chappell DC, Ishizaka N, et al. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 1998; 82(10): 1094–101
Paravicini TM, Chrissobolis S, Drummond GR, et al. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 2004; 35(2): 584–9
Didion SP, Faraci FM. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am J Physiol Heart Circ Physiol 2002; 282(2): H688–95
Beetsch JW, Park TS, Dugan LL, et al. Xanthine oxidase-derived superoxide causes reoxygenation injury of ischemic cerebral endothelial cells. Brain Res 1998; 786(1–2): 89–95
Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res 1999; 43(3): 521–31
Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD (P)H oxidase and endothelial nitric oxide synthase. Circulation 2002; 105(14): 1656–62
Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003; 111(8): 1201–9
Alp NJ, Channon KC. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 2004; 24: 1–9
Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in ApoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001; 103(9): 1282–8
Stralin P, Karlsson K, Johansson BO, et al. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol 1995; 15(11): 2032–6
Didion SP, Ryan MJ, Didion LA, et al. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 2002; 91(10): 938–44
Mayhan WG. Superoxide dismutase partially restores impaired dilatation of the basilar artery during diabetes mellitus. Brain Res 1997; 760(1–2): 204–9
Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 2001; 21(1): 2–14
Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep 2000; 2(1): 98–105
Leppala JM, Virtamo J, Fogelholm R, et al. Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke 1999; 30(12): 2535–40
Johansson BB. Hypertension mechanisms causing stroke. Clin Exp Pharmacol Physiol 1999; 26(7): 563–5
Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev 1998; 78(1): 53–97
Walder CE, Green SP, Darbonne WC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 1997; 28(11): 2252–8
Ito D, Murata M, Watanabe K, et al. C242T polymorphism of NADPH oxidase p22 PHOX gene and ischemic cerebrovascular disease in the Japanese population. Stroke 2000; 31(4): 936–9
Guzik TJ, West NE, Black E, et al. Functional effect of the C242T polymorphism in the NAD (P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation 2000; 102(15): 1744–7
Chamorro A, Vila N, Ascaso C, et al. Blood pressure and functional recovery in acute ischemic stroke. Stroke 1998; 29(9): 1850–3
Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 2000; 52(4): 639–72
Wei EP, Kontos HA, Patterson Jr JL. Vasoconstrictor effect of angiotensin on pial arteries. Stroke 1978; 9(5): 487–9
Haberl RL, Anneser F, Villringer A, et al. Angiotensin II induces endothelium-dependent vasodilation of rat cerebral arterioles. Am J Physiol 1990; 258 (6 Pt 2): H1840–6
Haberl RL. Role of angiotensin receptor subtypes in the response of rabbit brain arterioles to angiotensin. Stroke 1994; 25(7): 1476–9
Didion SP, Sigmund CD, Faraci FM. Impaired endothelial function in transgenic mice expressing both human renin and human angiotensinogen. Stroke 2000; 31(3): 760–4
Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest 1996; 97(8): 1916–23
Touyz RM, Chen X, Tabet F, et al. Expression of a functionally active gp91phox-containing neutrophil-type NAD (P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 2002; 90(11): 1205–13
Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke 2003; 34(8): 2038–42
Bosch J, Yusuf S, Pogue J, et al. Use of ramipril in preventing stroke: double blind randomised trial. BMJ 2002; 324(7339): 699–702
Chan PH. Role of oxidants in ischemic brain damage. Stroke 1996; 27(6): 1124–9
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22(9): 391–7
Liu TH, Beckman JS, Freeman BA, et al. Polyethylene glycolconjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol Heart Circ Physiol 1989; 256(2): H589–93
Kinouchi H, Epstein C, Mizui T, et al. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A 1991; 88(24): 11158–62
Yang G, Chan PH, Chen J, et al. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 1994; 25(1): 165–70
Kondo T, Reaume AG, Huang TT, et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci 1997; 17(11): 4180–9
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4(5): 399–415
Cook DA. Mechanisms of cerebral vasospasm in subarachnoid haemorrhage. Pharmacol Ther 1995; 66(2): 259–84
Sobey CG, Faraci FM. Subarachnoid haemorrhage: what happens to the cerebral arteries? Clin Exp Pharmacol Physiol 1998; 25(11): 867–76
Mori T, Nagata K, Town T, et al. Intracisternal increase of superoxide anion production in a canine subarachnoid hemorrhage model. Stroke 2001; 32(3): 636–42
Kim DE, Suh YS, Lee MS, et al. Vascular NAD (P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 2002; 33(11): 2687–91
Watanabe Y, Chu Y, Andresen JJ, et al. Gene transfer of extracellular superoxide dismutase reduces cerebral vasospasm after subarachnoid hemorrhage. Stroke 2003; 34(2): 434–40
Yamaguchi T, Sano K, Takakura K, et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 1998; 29(1): 12–7
Cherubini A, Polidori MC, Bregnocchi M, et al. Antioxidant profile and early outcome in stroke patients. Stroke 2000; 31(10): 2295–300
Leinonen JS, Ahonen JP, Lonnrot K, et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke 2000; 31(1): 33–9
Gale CR, Martyn CN, Winter PD, et al. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 1995; 310(6994): 1563–6
Yokoyama T, Date C, Kokubo Y, et al. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community. The Shibata Study. Stroke 2000; 31(10): 2287–94
Svetkey LP, Loria CM. Blood pressure effects of vitamin C: what’s the key question? Hypertension 2002; 40(6): 789–91
Kurl S, Tuomainen TP, Laukkanen JA, et al. Plasma vitamin C modifies the association between hypertension and risk of stroke. Stroke 2002; 33(6): 1568–73
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360(9326): 23–33
Yusuf S, Dagenais G, Pogue J, et al. Vitamin E supplementation and cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342(3): 154–60
Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation 2001; 104(22): 2638–40
Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342(3): 145–53
Lawes CM, Bennett DA, Feigin VL, et al. Blood pressure and stroke: an overview of published reviews. Stroke 2004; 35(3): 776–85
Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359(9311): 995–1003
Acknowledgements
Dr Sobey is supported by an R.D. Wright Fellowship (209160) from the National Health and Medical Research Council of Australia (NHMRC). Dr Drummond is supported by a NHMRC Peter Doherty Postdoctoral Fellowship (007044). Ms Paravicini is supported by an Australian Postgraduate Award from the Australian Government.
Dr Sobey and Dr Drummond hold equity in Radical Biotechnology Pty Ltd, a University of Melbourne-based company developing drugs to treat cardiovascular diseases. Dr Sobey is a Director of this company. Neither author receives commission or any direct income from this company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paravicini, T.M., Drummond, G.R. & Sobey, C.G. Reactive Oxygen Species in the Cerebral Circulation. Drugs 64, 2143–2157 (2004). https://doi.org/10.2165/00003495-200464190-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464190-00001