Abstract
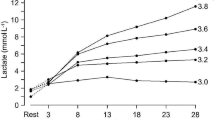
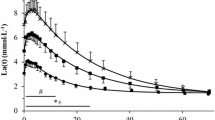
The maximal lactate steady state (MLSS) is defined as the highest blood lactate concentration (MLSSc) and work load (MLSSw) that can be maintained over time without a continual blood lactate accumulation. A close relationship between endurance sport performance and MLSSw has been reported and the average velocity over a marathon is just below MLSSw. This work rate delineates the low-to high-intensity exercises at which carbohydrates contribute more than 50% of the total energy need and at which the fuel mix switches (crosses over) from predominantly fat to predominantly carbohydrate. The rate of metabolic adenosine triphosphate (ATP) turnover increases as a direct function of metabolic power output and the blood lactate at MLSS represents the highest point in the equilibrium between lactate appearance and disappearance both being equal to the lactate turnover. However, MLSSc has been reported to demonstrate a great variability between individuals (from 2–8 mmol/L) in capillary blood and not to be related to MLSSw. The fate of enhanced lactate clearance in trained individuals has been attributed primarily to oxidation in active muscle and gluconeogenesis in liver. The transport of lactate into and out of the cells is facilitated by monocarboxylate transporters (MCTs) which are transmembrane proteins and which are significantly improved by training. Endurance training increases the expression of MCT1 with intervariable effects on MCT4. The relationship between the concentration of the two MCTs and the performance parameters (i.e. the maximal distance run in 20 minutes) in elite athletes has not yet been reported. However, lactate exchange and removal indirectly estimated with velocity constants of the individual blood lactate recovery has been reported to be related to time to exhaustion at maximal oxygen uptake.



Similar content being viewed by others
References
Brooks GA. Anaerobic threshold: review of the concept and direction for future research. Med Sci Sports Exerc 1985; 17: 31–5
Brooks GA, Fahey TD, White TP, et al. Exercise physiology. Mountain View (CA): Mayfield Publishing, 1996: 849
Billat VL, Demarle A, Slawinski J, et al. Physical and training characteristics of the top-class marathon runners. Med Sci Sports Exerc 2001; 33(12): 2089–97
Beneke R. Anaerobic threshold, individual anaerobic threshold, and maximal lactate steady state in rowing. Med Sci Sports Exerc 1995; 27: 863–7
Beneke R, Von Duvillard SP. Determination of maximal lactate steady-state response in selected sports events. Med Sci Sports Exerc 1996; 28: 241–6
Beneke R, Hütler M, Leithäuser R. Maximal lactate steady-state independent of performance. Med Sci Sports Exerc 2000; 32: 1135–9
Farrel PE, Wilmore JH, Coyle EF, et al. Plasma lactate accumulation and distance running performance. Med Sci Sports Exerc 1979; 11: 338–44
Billat VL, Dalmay F, Antonini MT, et al. A method for determining the maximal steady state of blood lactate concentration from two levels of submaximal exercise. Eur J Appl Physiol 1994; 69: 196–202
Billat VL. Use of blood lactate measurements for prediction of exercise performance and for control of training. Sports Med 1996; 22: 157–75
Lafontaine TP, Londeree BR, Spath WK. The maximal steady-state versus selected running events. Med Sci Sports Exerc 1981; 13: 190–2
Palmer GS, Borghouts LB, Noakes TD, et al. Metabolic and performance responses to constant-load vs variable-intensity exercise in trained cyclists. J Appl Physiol 1999; 87: 1186–96
Baldarini C, Guidetti L. A simple method for individual anaerobic threshold as predictor of max lactate steady state. Med Sci Sports Exerc 2000; 32: 1798–802
Jones AM, Carter H. The effect of endurance training on parameters of aerobic fitness. Sports Med 2000; 29: 373–86
Billat VL. Slow component and performance in endurance sports. Br J Sports Med 2000; 34: 83–5
Heck H, Mader A, Hess G, et al. Justification of the 4 mmol/L lactate threshold. Int J Sports Med 1985; 6: 117–30
Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc 2000; 32: 790–9
Faina M, Billat V, Squadrone R, et al. Anaerobic contribution to the time to exhaustion at the minimal exercise intensity at which maximal oxygen uptake occurs in elite cyclists, kayakists and swimmers. Eur J Appl Physiol Occup Physiol 1997; 76: 13–20
Bonen A. Lactate transporters (MCT proteins) in heart and skeletal muscles. Med Sci Sports Exerc 2000; 32: 778–89
Tesch PA, Wright JE. Recovery from short term intense exercise: its relation to capillary supply and blood lactate concentration. Eur J Appl Physiol 1983; 52: 98–103
Juel C. Lactate-proton co-transport in skeletal muscle. Physiol Rev 1997; 77: 321–58
Ingjer F. Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J Physiol 1979; 294: 419–32
Putman CT, Jones NL, Hultman E, et al. Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am J Physiol 1998; 275: E132–9
Pilegaard H, Bangsbo J, Richter EA, et al. Lactate transport studied in sarcolemmal giant vesicles from human muscle biopsies: relation to training status. J Appl Physiol 1994; 77: 1858–62
Garcia CK, Goldstein JL, Pathak RK, et al. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 1994; 76: 865–73
Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol 2001; 86: 6–11
Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol 1983; 244: E83–92
Eldridge FL, T’So L, Chang H. Relationship between turnover rate and blood concentration of lactate in normal dogs. J Appl Physiol 1974; 37: 316–20
Stanley WC, Lehman SL. A model for measurement of lactate disappearance with isotopic tracers in the steady state. Biochem J 1988 Dec 15; 256: 1035–8
Searle GL, Cavalieri RR. Determination of lactate kinetics in the human analysis of data from single injection vs continuous infusion methods. Proc Soc Exp Biol Med 1972; 139: 1002–6
Stanley WC, Gertz EW, Wisneski JA, et al. Systemic lactate kinetics during graded exercise in man. Am J Physiol 1985; 249: E595–602
Mazzeo RS, Brooks GA, Shoeller D, et al. Disposal of [1–13C] lactate during rest and exercise. J Appl Physiol 1986; 60: 232–41
Gladden LB. Muscle as a consumer of lactate. Med Sci Sports Exerc 2000; 32: 764–71
Margaria R, Edwards HT, Dill DB. The possible mechanism of contracting and paying the oxygen debt and the role of lactic acid in muscular contraction. Am J Physiol 1933; 106: 689–719
Margaria R, Cerretelli P, Mangili F. Balance and kinetics of anaerobic energy release during strenuous exercise in man. J Appl Physiol 1963; 19: 623–8
Issekutz Jr B, Shaw WA, Issekutz AC. Lactate metabolism in resting and exercising dogs. J Appl Physiol 1976; 40: 312–9
Brooks GA. The balance of carbohydrate and lipid use during sustained exercise: the cross-over concept. In: Whipp BJ, Sargeant AJ, editors. Physiological determinants of exercise tolerance in humans: studies in physiology, vol 4. Oxford: Portland Press, 1999: 61–76
Stanley WC, Wisneski EW, Gertz EW, et al. Glucose and lactate interrelations during moderate intensity exercise in man. Metabolism 1988; 37: 850–8
Spriet LL, Howlett RA, Heigenhauser GJF. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc 2000; 32: 756–63
Brooks GA. Are arterial, muscle and working limb lactate exchange data obtained on men at altitude consistent with the hypothesis of an intracellular lactate shuttle? In: Roach RC, editor. Hypoxia: into the next millennium. New York (NY): Kluwer Academic/Plenum Publishing, 1999: 184–204
Buckley JD, Scroop GC, Catcheside PG. Lactate disposal in resting trained and untrained forearm skeletal muscle during high intensity leg exercise. Eur J Appl Physiol 1993; 67(4): 360–6
Bergman BC, Wolfel EE, Butterfield GE, et al. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 1999; 87: 1684–96
McDermott JC, Elder GCB, Bonen A. Non-exercising muscle metabolism during exercise. Pfügers Arch 1991; 418: 301–7
Sumida K, Donovan CM. Lactate removal is not enhanced in non-stimulated perfused skeletal muscle after endurance training. J Appl Physiol 2001; 90(4): 1307–13
Stanley WC, Gertz EW, Wisneski JA, et al. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol 1986; 60: 1116–20
Gladden LB. Net lactate uptake during progressive steady-level contractions in canine skeletal muscle. J Appl Physiol 1991; 71: 514–20
Brooks GA, Dubouchaud H, Brown M, et al. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A 1999; 96: 1129–34
Poortmans JR, Delescaille-Vanden Bossche J, Leclercq R. Lactate uptake by inactive forearm during progressive leg exercise. J Appl Physiol 1978; 45: 835–9
Stainsby WN, Brooks GA. Control of lactic acid metabolism in contracting muscles and during exercise. Exerc Sport Sci Rev 1990; 18: 29–63
Hamann JJ, Kelley KM, Gladden LB. Effect of epinephrine on net lactate uptake by contracting skeletal muscle. J Appl Physiol 2001; 91: 2635–41
Watt MJ, Howlett KF, Febbraio MA, et al. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol 2001; 534(1): 269–78
Stainsby WN, Brechue WF, O’Drobinak DM. Regulation of muscle lactate production. Med Sci Sports Exerc 1991; 23: 907–11
Parolin ML, Spriet LL, Hultman E, et al. Effects of PDH activation by dichloroacetate in human skeletal muscle during exercise in hypoxia. Am J Physiol Endocrinol Metab 2000; 279: E752–61
Stanley WC, Connett RJ. Regulation of muscle carbohydrate metabolism during exercise. FASEB J 1991; 5: 2155–9
Nielsen HB, Clemmesen JO, Skak C, et al. Attenuated hepatosplanchnic uptake of lactate during intense exercise in humans. J Appl Physiol 2002; 92: 1677–83
Freund H, Oyono-Enguelle S, Heitz A, et al. Work rate-dependent lactate kinetics after exercise in humans. J Appl Physiol 1986; 61: 932–9
Freund H, Zouloumian P. Lactate after exercise in man: IV. physiological observations and model predictions. Eur J Appl Physiol Occup Physiol 1981; 46: 161–76
Messonnier L, Freund H, Feasson L, et al. Blood lactate exchange and removal abilities after relative high-intensity exercise: effects of training in normoxia and hypoxia. Eur J Appl Physiol 2001; 84: 403–12
Messonnier L, Freund H, Denis C, et al. Time to exhaustion at max is related to the lactate exchange and removal abilities. Int J Sports Med 2002; 23: 433–8
Brooks GA. Lactate: glycolytic product and oxidative substrate during sustained exercise in mamals-the lactate shuttle In: Gilles R, editor. Comparative physiology and biochemistry: current topics and trends. Vol. A: respiration-metabolism-circulation. Berlin: Springer-Verlag, 1985: 208–18
Brooks GA. Current concepts in lactate exchange. Med Sci Sports Exerc 1991; 23: 895–906
Barnard RJ, Edgerton VR, Furukawa T, et al. Histochemical, biochemical and contractile properties of red, white, and intermediate fibers. Am J Physiol 1971; 220: 410–4
Brooks GA, Gaesser GA. End points of lactate and glucose metabolism after exhausting exercise. J Appl Physiol 1980; 49: 1057–69
Brooks GA, Donovan CM. Effect of training on glucose kinetics during exercise. Am J Physiol 1982; 244: E505–12
Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol B Biochem Mol Biol 1998; 120: 89–107
Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol Endocrinol Metab 2001; 281: E794–802
Gladden LB. Lactic acid: new roles in a new millennium. Proc Nat Acad Sci U S A 2001, 98, 395–397
Hood DA. Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 2001; 90: 1137–57
Sahlin K, Fernström M, Svensson M, et al. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J Physiol 2002; 541(2): 569–74
Roth DA, Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 1990; 279: 377–85
McDermott JC, Bonen A. The regulation of myosin gene transcription in skeletal muscle: effects of altered functional demand. Can J Sport Sci 1991; 16: 210–22
McDermott JC, Bonen A. Endurance training increases skeletal muscle lactate transport. Acta Physiol Scand 1993; 147: 323–7
Poole RC, Halestrap AP. N-Terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochem J 1994; 303: 755–9
Juel C, Halestrap AP. Lactate transport in skeletal muscle: role and regulation of the monocarboxylate transporter. J Physiol 1999 Jun 15; 517 (Pt 3): 633–42
Fox JEM, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol 2000; 529: 285–93
Brooks GA, Brown MA, Butz CE, et al. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol 1999; 87: 1713–8
McCullagh KJA, Juel C, O’Brien MJ, et al. Chronic muscle stimulation increases lactate transport in rat skeletal muscle. Mol Cell Biochem 1996; 156: 51–7
McCullagh KJA, Poole RC, Halestrap AP, et al. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. Am J Physiol 1997; 273: R239–46
Pilegaard H, Terzis G, Halestrap A, et al. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol 1999; 276 (5 Pt 1): E843–8
Wilson MC, Jackson VN, Heddle C, et al. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem 1998; 273: 15920–6
Baker SK, Tarnopolsky MA, Bonen A. Expression of MCT-1 and MCT-4 in a patient with mitochondrial myopathy. Muscle Nerve 2001; 24: 394–8
Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999; 343 Pt 2: 281–99
Dubouchaud H, Butterfield GE, Wolfel EE, et al. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 2000; 278: E571–9
McDermott JC, Bonen A. Glyconeogenic and oxidative lactate utilization in skeletal muscle. Can J Physiol Pharmacol 1992; 70: 142–9
Pöso AR, Lampinen KJ, Räsänen LA. Distribution of lactate between red blood cells and plasma after exercise. Equine Vet J Suppl 1995; 18: 231–4
Räsänen LA, Lampinen KJ, Pösö AR. Responses of blood and plasma lactate and plasma purine concentratoins to maximal exercise and their relation to performance in standard bred trotters. Am J Vet Res 1995; 56: 1651–6
Poole RC, Halestrap AP. Transport of lactate and other mono-carboxylates across mammalian plasma membranes. Am J Physiol Cell Physiol 1993; 264: C761–82
Skelton MS, Kremer DE, Smith EW, et al. Lactate influx into red blood cells from trained and untrained human subjects. Med Sci Sports Exerc 1998; 30: 536–42
Väihkönen LK, Heinonen OJ, Hyyppä S. Lactate-transport activity in RBCs of trained and untrained individuals from four racing species. Am J Physiol Regul Integr Comp Physiol 2001; 281: R19–24
Saiki H, Margaria R, Cuttica F. Lactic acid production in submaximal work. Int Z Angew Physiol 1967; 24: 57–61
Wasserman K, McIlroy MB. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol 1964; 14: 844–52
Yoshida T. Effect of exercise duration during incremental exercise on the determination of anaerobic threshold and the onset of blood lactate accumulation. Eur J Appl Physiol 1984; 53: 196–9
Stegmann H, Kindermann W, Schnabel A. Lactate kinetics and individual anaerobic threshold. Int J Sports Med 1981; 2: 160–5
Schnabel A, Kindermann W, Schmitt WM, et al. Hormonal and metabolic consequences of prolonged running at the individual anaerobic threshold. Int J Sports Med 1982; 3: 163–8
Nagle F, Robinhold D, Howley E, et al. Lactic acid accumulation during running at submaximal aerobic demands. Med Sci Sports Exerc 1970; 2: 182–6
Londeree BR, Ames A. Maximal steady state versus state of conditioning. Eur J Appl Physiol 1975; 34: 269–78
Urhausen A. Individual anaerobic threshold and maximum lactate steady-state. Int J Sports Med 1993; 14: 134–9
Lajoie C, Laurencelle L, Trudeau F. Physiological responses to cycling for 60 minutes at maximal lactate steady state. Can J Appl Physiol 2000; 35: 250–61
Smith CGM, Jones AJ. The relationship between critical velocity, maximal lactate steady-state velocity and lactate turnpoint velocity in runners. Eur J Appl Physiol 2001; 85: 19–26
McLellan TM, Jacobs I. Reliability, reproducibility and validity of the individual anaerobic threshold. Eur J Appl Physiol 1993; 67: 125–31
Bacon L, Kern M. Evaluating a test protocol for predicting maximum lactate steady-state. J Sports Med Phys Fitness 1999; 39: 300–8
Mocellin R, Heusgen M, Korsten-Reck U. Maximal steady state blood lactate levels in 11-year-old boys. Eur J Pediatr 1990; 149: 771–3
Billat V, Gratas-Delamarche A, Monnier M, et al. A test to approach maximal lactate steady-state in 12-year old boys and girls. Arch Physiol Biochem 1995; 103: 65–72
di Prampero PE, Atchou G, Brückner JC, et al. The energetics of endurance running. Eur J Appl Physiol 1986; 55: 259–6
Hoogeveen AR, Hoogsteen J, Schep G. The maximal lactate steady state in elite endurance athletes. Jpn J Physiol 1997; 47: 481–5
Myburgh KH, Viljoen A, Tereblanche S. Plasma lactate concentrations for self-selected maximal effort lasting 1 h. Med Sci Sports Exerc 2001; 33: 152–6
Harnish CR, Swensen TC, Pate RR. Methods for estimating the maximal lactate steady state in trained cyclists. Med Sci Sports Exerc 2001; 33: 1052–5
Foxdal P, Sjödin B, Rudstam H, et al. Lactate concentration differences in plasma, whole blood, capillary finger blood and erythrocytes during submaximal graded exercise in humans. Eur J Appl Physiol 1990; 61: 218–22
Smith EW, Skelton MS, Kremer DE, et al. Lactate distribution in the blood during steady-state exercise. Med Sci Sports Exerc 1998; 30: 1424–9
Boning D. Differences between whole blood and plasma lactate concentrations have to be considered when comparing various studies. Med Sci Sports Exerc 2001; 33: 1411–2
Beneke R, Heck H, Schwarz V, et al. Maximal lactate steady-state during the second decade of age. Med Sci Sports Exerc 1996; 28: 1474–8
Billat VL, Slawinski J, Danel M, et al. Effect of free versus constant-pace on performance and oxygen kinetics in running. Med Sci Sports Exerc 2001; 33: 2082–8
Cottin F, Papelier Y, Durbin F, et al. Effect of fatigue on spontaneous velocity variations in human middle-distance running: use of short-term Fourier transformation. Eur J Appl Physiol 2002; 87: 17–27
Zamparo P, Perini R, Peano C, et al. The self selected speed of running in recreational long-distance runners. Int J Sports Med 2001; 22: 598–604
Glass C, Knowlton RG, Sajabi PB, et al. The effect of exercise induced glycogen depletion on the lactate, ventilatory and electromyographic thresholds. J Sports Med Phys Fitness 1997; 37: 32–40
Genevely H, Stamford BA. Effects of prolonged warm-up exercise above and below anaerobic threshold on maximal performance. Eur J Appl Physiol Occup Physiol 1982; 48: 323–30
Mognoni P, Sirtori MD, Lorenzi F, et al. Physiological responses during prolonged exercise at the power output corresponding to the blood lactate threshold. Eur J Appl Physiol 1990; 60: 239–43
Oyono-Enguelle S, Heitz A, Marbach J, et al. Blood lactate during constant-load exercise at aerobic and anaerobic thresholds. Eur J Appl Physiol 1990; 60: 321–30
Sahlin K, Tonkonogi M, Söderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand 1998; 162: 261–6
Shulman RG, Rothman DL. The ‘glycogen shunt’ in exercising muscle: a role for glycogen in muscle energetics and fatigue. Proc Natl Acad Sci U S A 2001; 98: 457–61
O’Brien MJ, Viguie CA, Mazzeo RS, et al. Carbohydrate dependence during marathon running. Med Sci Sports Exerc 1993; 25: 1009–17
Helge JW, Watt PW, Richter EA, et al. Fat utilization during exercise: adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylg-lycerol in humans. J Physiol 2001; 537 (Pt 3): 1009–20
Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the ‘crossover’ concept. J Appl Physiol 1994; 76: 2253–61
Gladden LB. Lactate uptake by skeletal muscle. Exerc Sport Sci Rev 1989; 17: 115–55
Ettema JH. Limits of human performance and energy production. Int Z Angew Physiol 1966; 22: 45–54
Hill DW. The critical power concept: a review. Sports Med 1993; 16: 237–54
Morton H, Billat V. Maximal endurance time at max. Med Sci Sports Exerc 2000; 32: 1496–504
Monod H, Scherrer J. The work capacity of synergy muscular group. Ergonomics 1965; 8: 339–50
Billat V, Renoux JC, Pinoteau, et al. Times to exhaustion at 100% of velocity at max and modelling of the velocity-time relationship in elite long-distance runners. Eur J Appl Physiol Occup Physiol 1994; 69: 271–3
Gaesser GA, Wilson LA. Effects of continuous and interval training on the parameters of the power-endurance time relationship for high-intensity exercise. Int J Sports Med 1988; 9: 417–21
Housh DJ, Housh TJ, Bauge SM. The accuracy of the critical power test for predicting time to exhaustion during cycle ergometry. Ergonomics 1989; 32: 997–1004
Jenkins DG, Quigley BM. The Y-intercept of the critical power duration as a measure of anaerobic work capacity. Ergonomics 1991; 34: 13–22
McLellan TM, Cheung KS. A comparative evaluation of the individual anaerobic threshold at the onset of muscular exercise in man. Ergonomics 1992; 8: 49–54
Pepper ML, Housh TJ, Johnson GO. The accuracy of the critical velocity test for predicting time to exhaustion during treadmill running. Int J Sports Med 1992; 13: 121–4
Morton RH. A 3-parameter critical power model. Ergonomics 1996; 39: 611–9
Gaesser GA, Carnevale TJ, Garfinkel A, et al. Estimation of critical power with nonlinear and linear models. Med Sci Sports Exerc 1995; 27: 1430–8
Le Chevalier JM, Vandewalle H, Chatard JC, et al. Relationship between the 4 mM running velocity, the time-distance relationship and the Légéer-Boucher’s test. Arch Int Physiol Biochim Biophys 1989; 97: 355–60
Billat V, Binsse V, Petit B, et al. High level runners are able to maintain a steady-state below V̇O2max in all-out run over their critical velocity. Arch Physiol Biochem 1998; 106: 38–45
Billat VL, Blondel N, Berthoin S. Determination of the velocity associated with the longest time to exhaustion at maximal oxygen uptake. Eur J Appl Physiol 1999; 80: 159–61
Whipp BJ, Ozyener F. The kinetics of exertional oxygen uptakes: assumptions and inferences. Med Sport 1998; 51: 139–49
Carter H, Pringle JS, Jones AM, et al. Oxygen uptake kinetics during treadmill running across exercise intensity domains. Eur J Appl Physiol 2002; 86: 347–54
Ozyener F, Rossiter HB, Ward SA, et al. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 2001; 533 (Pt 3): 891–902
Poole DC, Ward SA, Gardner GW, et al. Metabolic and respiratory profile of heavy and severe exercise. Eur J Appl Physiol 1988; 31: 1265–79
Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sports Sci Rev 1996; 24: 35–70
Xu F, Rhodes EC. Oxygen uptake kinetics during exercise. Sports Med 1999; 27: 313–27
Tokmakaidis SP, Léger LA. Comparison of mathematically determined blood lactate and heart rate ‘threshold’ points and relationship with performance. Eur J Appl Physiol 1992; 64: 309–17
Beneke R, Leithauser RM, Hutler M. Dependence of the maximal lactate steady state on the motor pattern of exercice. Br J Sports Med 2001; 35: 192–6
Gobatto CA, de Mello MA, Sibuya CY, et al. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol 2001; 130: 21–7
Lokkegaard J, Pedersen PK, Juel C, et al. Individual variations in maximal lactate steady-state and their relationship with muscle buffering capacity and lactate transporters. Med Sci Sports Exerc 2001; 33Suppl. 30: S15
Donovan CM, Pagliassotti MJ. Endurance training enhances lactate clearance during hyperlactatemia. Am J Physiol Endocrinol Metab 1989; 257: E782–9
Pilegaard H, Juel C, Wibrand F. Lactate transport studied in sarcolemmal giant vesicles from rat: effect of training. Am J Physiol 1993; 264: R156–60
Baker SK, McCullagh KJA, Bonen A. Training intensity-dependent and tissue-specific increases in lactate uptake and MCT-1 in heart and muscle. J Appl Physiol 1998; 84: 987–94
Eydoux N, Py G, Lambert K, et al. Training does not protect against exhaustive exercise-induced lactate transport capacity alterations. Am J Physiol Metab 2000; 278: E1045–52
Pilegaard H, Bangsbo J, Richter EA, et al. Lactate transport studied in sarcolemmal giant vesicles from human muscle biopsies: relation to training status. J Appl Physiol 1994; 77: 1858–62
Pilegaard H, Domino K, Nolnd T, et al. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol 1999; 276: E255–61
Juel C. Muscle pH regulation: role of training. Acta Physiol Scand 1998; 162: 359–66
Green H, Halestrap A, Mockett C, et al. Increases in muscle MCT are associated with reductions in muscle lactate after a single exercise session in humans. Am J Physiol Endocrinol Metab 2002; 282: E154–60
Phillips SM, Green HJ, Tarnopolsky MA, et al. Increased clearance of lactate after short-term training in men. J Appl Physiol 1995; 79(6): 1862–9
Bonen A, McCullagh KJA, Putman CT, et al. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am J Physiol Endocrinol Metab 1998; 274: E102–7
Bergman BC, Butterfield GE, Wolfel EE, et al. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol 1999 Jul; 277 (1 Pt 1): E81–92
Evertsen F, Medbo JI, Bonen A. Effect of training intensity on muscle lactate transporters and lactate threshold of cross-country skiers. Acta Physiol Scand 2001; 173: 195–205
Acknowledgements
This study was supported by grants from la Fondation d’Entreprise gaz de France (France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billat, V.L., Sirvent, P., Py, G. et al. The Concept of Maximal Lactate Steady State. Sports Med 33, 407–426 (2003). https://doi.org/10.2165/00007256-200333060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200333060-00003