Abstract
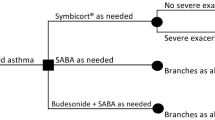
Introduction: Budesonide/formoterol (Symbicort®) Maintenance And Reliever Therapy (SMART) is an effective and well tolerated treatment option for patients with asthma. We compared the cost effectiveness from a societal perspective of this one-inhaler regimen with that of maintenance salmeterol/fluticasone propionate (Seretide®) plus salbutamol (albuterol) as needed (Seretide® Fixed Combination [SFC]).
Study design: A cost-effectiveness analysis was performed based on effectiveness and resource-utilisation data collected prospectively in a randomised, 12-month study performed in 2143 patients in 16 countries. Resource utilisation data were pooled and unit costs (€, year 2003 values) from Italy, France, the UK and Germany were used to generate estimates of direct and total costs per patient per year and cost per severe exacerbation avoided.
Methods: Adolescents and adults with asthma (n = 2143; mean forced expiratory volume in 1 second [FEV1] 73% predicted; mean inhaled corticosteroid [ICS] dose 884 µg/day) were randomised to SMART or SFC. The effectiveness measure used was the number of severe exacerbations per patient per year. Direct costs included medication use (budesonide/formoterol 160µg/4.5µg or salmeterol/fluticasone 50µg/100µg, 50µg/250µg or 50µg/500µg plus salbutamol) and nonmedication-related resource use, including days in hospital, emergency room visits, specialist or primary care physician visits and other healthcare provider contacts. Indirect costs, including the number of days when the patient or their carer was unable to attend to their normal daily activities, were also assessed. The study assumed a European societal perspective (i.e. including direct and indirect costs).
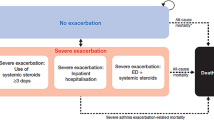
Results: Treatment with SMART resulted in significantly fewer severe exacerbations per patient per year compared with SFC (0.24 vs 0.31 events per patient per year; p = 0.0025). Resource use was low in both groups. Medication costs accounted for the majority of the total costs. The increased effectiveness of SMART was achieved at a reduced or similar cost compared with SFC. SMART dominated when German unit costs were applied (i.e. there was a statistically significant reduction in both costs and number of exacerbations). In all other countries, the incremental cost-effectiveness ratios showed that there was a reduction in mean total cost per exacerbation avoided; however, this difference was not statistically significant.
Conclusion: This analysis demonstrates that, compared with SFC, SMART may be cost effective from a societal perspective for the treatment of patients with asthma in Italy, Germany, France and the UK. SMART provided a reduction in the number of severe exacerbations per patient per year, at no statistically significant increase in cost — or even at a lower cost — compared with SFC plus as-needed reliever salbutamol.








Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Global Initiative for Asthma. Global strategy for asthma management and prevention. National Institutes of Health publication No. 02-3659 (updated 2004). Bethesda (MD): National Institutes of Health, 2004 [online]. Available from URL: http://www.ginasthma.org [Accessed 2005 Jul 6]
Stempel DA, Stoloff SW, Carranza Rosenzweig JR, et al. Adherence to asthma controller medication regimens. Respir Med 2005 Oct; 99 (10): 1263–1267
Greening AP, Ind PW, Northfield M, et al. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994 Jul; 344 (8917): 219–224
Pauwels RA, Lofdahl C-G, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1997 Nov; 337 (2): 1405–1411
Woolcock A, Lundback B, Ringdal N, et al. Comparison of addition of salmeterol to inhaled steroids with doubling the dose of inhaled steroids. Am J Respir Crit Care Med 1996 May; 153 (5): 1481–1488
Aalbers R, Backer V, Kava IT, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Curr Med Res Opin 2004 Feb; 20 (2): 225–240
Bateman ED, Bantje TA, Joao Gomes M, et al. Combination therapy with single inhaler budesonide/formoterol compared with high dose off luticasone propionate alone in patients with moderate persistent asthma. Am J Respir Med 2003; 2 (3): 275–281
Buhl R, Creemers JPHM, Vondra V, et al. Once-daily budesonide/formoterol in a single inhaler in moderate persistent asthma. Respir Med 2003 Apr; 97 (4): 323–330
FitzGerald JM, Sears MR, Boulet L-P, et al. Adjustable maintenance dosing with budesonidelformoterol reduces asthma exacerbations compared with traditional fixed dosing: a five-month multicentre Canadian study. Can Respir J 2003 Nov–Dec; 10 (8): 427–434
Kavum M, Melamed J, Gross G, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2000 Jun; 105 (6 Pt 1): 1108–1116
Lalloo UJ, Malolepszy J, Kozma D, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest 2003 May; 123 (5): 1480–1487
Shapiro G, Lurrry W, Wolfe J, et al. Combined salmeterol 50µg and fluticasone propionate 250 µg in the Diskus device for the treatment of asthma. Am J Respir Crit Care Med 2000 Feb; 161 (2 Pt 1): 527–534
Stallberg B, Olsson P, Jorgensen LA, et al. Budesonide/formoterol adjustable maintenance dosing reduces asthma exacerbations versus fixed dosing. Int J Clin Pract 2003 Oct; 57 (8): 656–661
Zetterstram O, Bubl R, Mellem H, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Eur Respir J 2001 Aug; 18 (2): 262–268
Gibson PG. Teaching old drugs new tricks: asthma therapy adjusted by patient perception or noninvasive markers. Eur Respir J 2005 Mar; 25 (3): 397–399
Barnes PJ. A single inhaler for asthma? Am J Respir Crit Care Med 2005 Jan 15; 171 (2): 95–96
O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005 Jan; 171 (2): 129–136
Rabe KF, Pizzichini E, Ställberg B, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild to moderate asthma: a randomized, double-blind trial. Chest 2006 Feb; 129 (2): 246–256
Scicchitano R, Aalbers R, Ukena D, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin 2004 Sep; 20 (9): 1403–1418
Rosenhall L, Borg S, Andersson F, et al. Budesonide/formoterol in a single inhaler (Symbicort®) reduces healthcare costs compared with separate inhalers in the treatment of asthma over 12 months. Int J Clin Pract 2003 Oct; 57 (8): 662–668
Jonsson B, Berggren F, Svensson K, et al. An economic evaluation of combination treatment with budesonide and formoterol in patients with mild-to-moderate persistent asthma. Respir Med 2004 Nov; 98 (11): 1146–1154
Lindgren B, Sears MR, Campbell M, et al. Cost-effectiveness of formoterol and salbutamol as asthma reliever medication in Sweden and Spain. Int J Clin Pract 2005 Jan; 59 (1): 62–68
Vogelmeier C, D’Urzo A, Pauwels R, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J 2005 Nov; 26 (5): 819–828
Medical Products Agency, Uppsala, Sweden. Seretide Diskus mite, Seretide Diskus, Seretide Diskus forte (salmeterol/flutikason) [online]. Available from URL: http://www.lakemedelsverket.se/Tpl/MonographyPage_597.aspx [Accessed 2006 May 25]
Medical Products Agency, Uppsala, Sweden. Symbicort Turbubaler/Symbicort mite Turbuhaler [online]. Available from URL: http://www.lakernedelsverket.set/Tpl/MonographyPage_604.aspx [Accessed 2006 May 25]
National formulary 2004, Official Gazette No.4, 2003 Jan 7th ed. Rome: Italian Ministry of Health, 2004
Groupement pour l’Elaboration et la Realisation de Statistiques. Paris: 2004 [online]. Available from URL: http://www.giegers.fr/ [Accessed 2005 Mar]
Monthly Index of Medical Specialties (MIMS) 2004 Jun ed. London: Haymarket Publications, 2004
Lauer-Taxe: WINAPO® Lauer-Taxe, Lauer-Fischer, Germany [online]. Available from URL: http://www.arz.de/LAUERFISCHER/default.htm [Accessed 2004 Jul]
Arvidsson P, Mellen A, Palmqvist M, et al. Equivalent therapeutic ratio of salbutamol given by Turbuhaler and Diskus. Respir Med 2000 Jun; 94 (6): 574–577
IMS Health, IMS MIDAS Quantum MAT/Q2/04 [database]. London: IMS Health, 2005
Italian Ministry of Health. Official gazette No. 209, 1997 Sept 8. Ordinary Supplement, 1997 March 12
MEDTAP. Database of international unit costs for economic evaluations in health care. London: MEDTAP International Inc, 2002 Dec 18 [online]. Available from URL: http://www.medtap.com/products/unitcost.cfm [Accessed 2006 May 30]
NHS reference costs 2003 and national tariff 2004, Appendix 4. National schedule of reference costs: NHS Trusts and Primary Care Trusts combined; TCCS, Service Code CCI [online]. Available from URL: http://www.dh.gov.uk/assetRoot/04/07/01/16/04070116.xls [Accessed 2005 Jul 6]
PKV, Zahlenbericht 2002/2003, PKV Verband der privaten Krankenversicherung e.V., Köln, Germany [online]. http://www.pkv.de/downloads/Zb031.pdf [Accessed 2006 May 24]
Potena A, Ballerin L, Putinati S, et al. The intensive respiratory care units in Italy: analysis of clinical activity and coding system. Rev Pathol Respir System 2002; 17: 96–105
NHS reference costs 2003, HRG code D22 [online]. Available from URL: http://www.nhselect.org.uk/downloads/nhselect_price_list_2005-06a.htm [Accessed 2005 Jul 6]
Arrêté du 2 Août 2004 fixant les tarifs de référence nationaux par activité. Annexe 1 titre II et III. Journal Officiel de la République Française (JORF) du 22 août 2004 [online]. Available from URL: http://www.legifrance.gouv.fr/ [Accessed 2006 May 25]
Curtis L, Netten A. Unit costs of health and social care 2004. Canterbury (UK): PSSRU, University of Kent at Canterbury, 2004
Deutsche Krankenhausgesellschaft, DKG-NTIBG Tariff, 2002/2003. Germany: Kohlhammer Verlag, 2003
Nomenclature Générale des Actes Professionnels, Union des Caisses Nationales de Sécurité Sociale (UCANSS), 2004 [online]. Available from URL: http://www.ucanss.fr/ucanss.nsf/ [Accessed 2005 Jul 6]
Kölner Kommentar zum EBM. Kommentierung zum einheitlichen Bewertungsmaßstab Manfred Moewes, Erhard Effer, Rainer Hess. Köln: Deutscher Ärzteverlag, 1994 (updated October 2002)
Lucioni C, Mangrella M, Mazzi S, et al. Treatment of patients with asthma with a fixed combination of budesonide and formoterol: a pharmacoeconomic evaluation vs some therapeutic alternatives. Pharmacoeconomics Italian Res Articles 2002; 4 (1): 15–23
Bondonio P, Eandi M. The times and costs of work associated to the pharmacological treatment: analysis of some cost-saving opportunities. Farmeconomia 1995; 2: 36–45
Démarches des soins infirmiers. Paris: CNAMTS (Caisse nationale d’ Assurance Maladie des Travailleurs Salariés), 2002 [online]. Available from URL: http://www.ameli.fr/ [Accessed 2006 May 30]
INSEE Première No 1007. France: INSEE (National Institute for Statistics and Economic Studies), 2005 Mar [online]. Available from URL: http://www.insee.fr/ [Accessed 2006 May 30]
United Kingdom National Statistics. New earning survey 2003 [online]. Available from URL: http://www.statistics.gov.uk/pdfdir/nesl003.pdf [Accessed 2005 Jul 6]
Statistisches Jahrbuch für die Bundesrepublik Deutschland 2003. Wiesbaden: Statistisches Bundessamt (Federal Statistics Office), 2003
Tatiersfield AE, Postma DS, Barnes PI, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med 1999 Aug; 160 (2): 594–599
Mellis eM, Peat JK, Woolcock AI. The cost of asthma: can it be reduced? Pharmacoeconomics 1993 Mar; 3 (3): 205–219
Weiss KB, Sullivan SD. The health economics of asthma and rhinitis: I. Assessing the economic impact. J Allergy Clin Immunol 2001 Jan; 107 (1): 3–8
Hufford MR, Shiffman S. Assessment methods for patient-reported outcomes. Dis Manage Health Outcomes 2003; 11 (2), 77–86
Butz AM, Donithan M, Bollinger ME, et al. Monitoring nebulizer use in children: comparison of electronic and asthma diary data. Ann Allergy Asthma Immunol 2005 Mar; 94 (3): 360–365
Sullivan SD, Liljas B, Buxton M, et al. Design and analytic considerations in determining the cost-effectiveness of early intervention in asthma from a multinational clinical trial. Control Clin Trials 2001 Aug; 22 (4): 420–437
Jönsson B, Weinstein MC. Economic evaluation alongside multinational clinical trials: study considerations for GUSTO IIb. Int J Technol Assess Health Care 1997; 13: 49–58
Reed SD, Anstrom KJ, Bakhai A, et al. Conducting economic evaluations alongside multinational clinical trials: towards a research consensus. Am Heart J 2005; 149: 434–443
Buxton MJ, Sullivan SD, Andersson LF, et al. Country-specific cost-effectiveness of early intervention with budesonide in mild asthma. Eur Respir J 2004; 24: 568–574
Ramsey S, Willke R, Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005 Sep–Oct; 8 (5), 521–533
Acknowledgements
Gunnar Johansson and Claus Vogelmeier were involved in the clinical study and in the interpretation of the data and the writing of the manuscript. Emma Andreasson was involved in the design and implementation of the study, interpretation of data and development of the manuscript. Per Larsson contributed to the study design, statistical analysis and interpretation of results. All authors had free and unlimited access to the clinical study report and statistical reports. All authors were involved in the decision to submit the manuscript. All authors made final decisions on all aspects of the manuscript and hence are in agreement with, and approve, the final version of the submitted manuscript.
AstraZeneca provided financial support for this study as recommended by national guidelines and approved by local ethics committees. AstraZeneca provided study medication, clinical trial materials and study monitors, and paid other study-related costs.
Emma Andreasson was employed by AstraZeneca, Lund, Sweden, at the time this article was written.
Gunnar Johansson does not hold any stocks or shares in any organisation that could gain or lose financially from the results of this study. He has conducted clinical trials for AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline and Merck, Sharpe & Dohme and has also been an invited speaker for these companies during the last 5 years. Emma Andreasson was an AstraZeneca employee at the time of the study. Per Larsson is an AstraZeneca employee. During the last 5 years, Claus Vogelmeier has presented at symposia sponsored by Altana, AstraZeneca, Aventis, Bayer, Boehringer Ingelheim, GlaxoSmithKline and Merck Darmstadt. He has also been a paid consultant for Altana, AstraZeneca, Bayer, Boehringer Ingelheim and GlaxoSmithKline.
We acknowledge the contribution of Deirdre Carman from Adelphi Communications, who provided medical writing services on behalf of AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johansson, G., Andreasson, E.B., Larsson, P.E. et al. Cost Effectiveness of Budesonide/Formoterol for Maintenance and Reliever Therapy versus Salmeterol/ Fluticasone plus Salbutamol in the Treatment of Asthma. Pharmacoeconomics 24, 695–708 (2006). https://doi.org/10.2165/00019053-200624070-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200624070-00008