Abstract
Genetic and experimental evidence points to amyloid-β (Aβ) peptide as the culprit in Alzheimer’s disease pathogenesis. This protein fragment abnormally accumulates in the brain cortex and hippocampus of patients with Alzheimer’s disease, and self-aggregates to form toxic oligomers causing neurodegeneration.
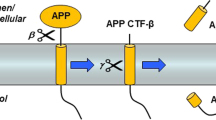
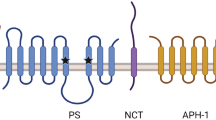
Aβ is heterogeneous and produced from a precursor protein (amyloid precursor protein [APP]) by two sequential proteolytic cleavages that involve β- and γ-secretases. This latter enzyme represents a potentially attractive drug target since it dictates the solubility of the generated Aβ fragment by creating peptides of various lengths, namely Aβ40 and Aβ42, the longest being the most aggregating. γ-Secretase comprises a molecular complex of four integral membrane proteins — presenilin,nicastrin, APH-1 and PEN-2 — and its molecular mechanism remains under extensive scrutiny. The ratio of Aβ42 over Aβ40 is increased by familial Alzheimer’s disease mutations occurring in the presenilin genes or in APP, near the γ-secretase cleavage site.
Potent γ-secretase inhibitors have been identified by screening drug libraries or by designing aspartyl protease transition-state analogues based on the APP substrate cleavage site. Most of these compounds are not specific for γ-secretase cleavage of APP, and equally inhibit the processing of other γ-secretase substrates, such as Notch and a subset of cell-surface receptors and proteins involved in embryonic development, haematopoiesis, cell adhesion and cell/cell contacts. Therefore, current research aims at finding compounds that show selectivity for APP cleavage, and particularly that inhibit the formation of the aggregating form, Aβy42. Compounds that target the substrate docking site rather than the enzyme active site are also being investigated as an alternative strategy. The finding that some NSAID analogues preferentially inhibit the formation of Aβ42 over Aβ40 and do not affect Notch processing has opened a new therapeutic window. The progress in design of selective inhibitors as well as recent results obtained in animal studies prove that γ-secretase remains among the best targets for the therapeutic control of amyloid build-up in Alzheimer’s disease. The full understanding of γ-secretase regulation may yet uncover new therapeutic leads.






Similar content being viewed by others
References
Bertram L, Tanzi RE. Dancing in the dark? The status of late-onset Alzheimer’s disease genetics. J Mol Neurosci 2001; 17(2): 127–36
Voisin T, Reynish E, Portet F, et al. What are the treatment options for patients with severe Alzheimer’s disease? CNS Drugs 2004; 18(9): 575–83
Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 1984; 120(3): 885–90
Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 1985; 82(12): 4245–9
Kang J, Lemaire HG, Unterbeck A, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987; 325(6106): 733–6
Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 2001; 81(2): 741–66
Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Aβ amyloid peptides. Peptides 2002; 23(7): 1285–97
Sisodia SS, Koo EH, Beyreuther K, et al. Evidence that β-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science 1990; 248(4954): 492–5
Hardy J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 1997; 20(4): 154–9
St George-Hyslop PH. Genetic factors in the genesis of Alzheimer’s disease. Ann N Y Acad Sci 2000; 924: 1–7
Bertram L, McQueen M, Mullin K, et al. The AlzGene database: Alzheimer research forum [online]. Available from URL: http://www.alzforum.org/res/com/mut/pre/default.asp [Accessed 24/3/06]
Holsinger RM, McLean CA, Beyreuther K, et al. Increased expression of the amyloid precursor β-secretase in Alzheimer’s disease. Ann Neurol 2002; 51(6): 783–6
Fukumoto H, Cheung BS, Hyman BT, et al. β-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 2002; 59(9): 1381–9
Yang LB, Lindholm K, Yan R, et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 2003; 9(1): 3–4
McLean CA, Cherny RA, Fraser FW, et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 1999; 46(6): 860–6
Nunan J, Shearman MS, Checler F, et al. The C-terminal fragment of the Alzheimer’s disease amyloid protein precursor is degraded by a proteasome-dependent mechanism distinct from γ-secretase. Eur J Biochem 2001; 268(20): 5329–36
Weidemann A, Eggert S, Reinhard FB, et al. A novel ε-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry 2002; 41(8): 2825–35
Sastre M, Steiner H, Fuchs K, et al. Presenilin-dependent γ-secretase processing of β-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep 2001; 2(9): 835–41
Yu C, Kim SH, Ikeuchi T, et al. Characterization of a presenilin-mediated amyloid precursor protein carboxyl-terminal fragment γ: evidence for distinct mechanisms involved in γ-secretase processing of the APP and Notchl transmembrane domains. J Biol Chem 2001; 276(47): 43756–60
Gu Y, Misonou H, Sato T, et al. Distinct intramembrane cleavage of the β-amyloid precursor protein family resembling γ-secretase-like cleavage of Notch. J Biol Chem 2001; 276(38): 35235–8
Cao X, Sudhof TC. Dissection of amyloid-β precursor protein-dependent transcriptional transactivation. J Biol Chem 2004; 279(23): 24601–11
Buxbaum JD, Liu KN, Luo Y, et al. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem 1998; 273(43): 27765–7
Lammich S, Kojro E, Postina R, et al. Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A 1999; 96: 3922–7
Asai M, Hattori C, Szabo B, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP α-secretase. Biochem Biophys Res Commun 2003; 301(1): 231–5
Vassar R, Bennett BD, Babu-Khan S, et al. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999; 286(5440): 735–41
Hussain I, Powell D, Howlett DR, et al. Identification of a Novel Aspartic Protease (Asp 2) as β-Secretase. Mol Cell Neurosci 1999; 14(6): 419–27
Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 1999; 402(6761): 537–40
Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 1999; 402(6761): 533–7
Lin X, Koelsch G, Wu S, et al. Human aspartic protease memapsin 2 cleaves the β-secretase site of β-amyloid precursor protein. Proc Natl Acad Sci U S A 2000; 97: 1456–60
Cai H, Wang Y, McCarthy D, et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci 2001 Mar; 4(3): 233–4
Luo Y, Bolon B, Kahn S, et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci 2001 Mar; 4(3): 231–2
Roberds SL, Anderson J, Basi G, et al. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet 2001; 10: 1317–24
Lichtenthaler S, Wang R, Grimm H, et al. Mechanism of the cleavage specificity of Alzheimer’s disease γ-secretase identified by phenylalanine-scanning mutagenesis of the transmembrane domain of the amyloid precursor protein. Proc Natl Acad Sci U S A 1999; 96: 3053–8
Wang R, Sweeney D, Gandy SE, et al. The profile of soluble amyloid β protein in cultured cell media: detection and quantification of amyloid β protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem 1996; 271(50): 31894–902
Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochem Biophys Acta 2000; 1502: 172–87
Murphy MP, Hickman LJ, Eckman CB, et al. γ-Secretase, evidence for multiple proteolytic activities and influence of membrane positioning of substrate on generation of amyloid β peptides of varying length. J Biol Chem 1999; 274(17): 11914–23
Beher D, Wrigley JD, Owens AP, et al. Generation of C-terminally truncated amyloid-β peptides is dependent on γ-secretase activity. J Neurochem 2002; 82(3): 563–75
Esh C, Patton L, Kalback W, et al. Altered APP processing in PDAPP (Val717→Phe) transgenic mice yields extended-length Aβ peptides. Biochemistry 2005 Oct 25; 44(42): 13807–19
Jarrett JT, Lansbury Jr PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 1993; 73(6): 1055–8
Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, et al. Longer forms of amyloid β protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J Neurosci 2005 Jan 12; 25(2): 436–45
Zhao G, Mao G, Tan J, et al. Identification of a new presenilin-dependent zeta-cleavage site within the transmembrane domain of amyloid precursor protein. J Biol Chem 2004 Dec 3; 279(49): 50647–50
Zhao G, Cui MZ, Mao G, et al. γ-Cleavage is dependent on zeta-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem 2005 Nov 11; 280(45): 37689–97
Sato T, Tanimura Y, Hirotani N, et al. Blocking the cleavage at midportion between γ- and ε-sites remarkably suppresses the generation of amyloid β-protein. FEBS Lett 2005 May 23; 579(13): 2907–12
Funamoto S, Morishima-Kawashima M, Tanimura Y, et al. Truncated carboxyl-terminal fragments of β-amyloid precursor protein are processed to amyloid β-proteins 40 and 42. Biochemistry 2004 Oct 26; 43(42): 13532–40
Li YM, Lai MT, Xu M, et al. Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A 2000; 97(11): 6138–43
Shearman MS, Beher D, Clarke EE, et al. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid β-protein precursor γ-secretase activity. Biochemistry 2000; 39(30): 8698–704
Wolfe MS, Citron M, Diehl TS, et al. A substrate-based difluoro ketone selectively inhibits Alzheimer’s γ-secretase activity. J Med Chem 1998; 41(1): 6–9
Tian G, Sobotka-Briner CD, Zysk J, et al. Linear non-competitive inhibition of solubilized human γ-secretase by pepstatin A methylester, L-685458, sulfonamides and benzodiazepines. J Biol Chem 2002; 277(35): 31499–505
De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 1998; 391(6665): 387–90
Herreman A, Serneels L, Annaert W, et al. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol 2000; 2(7): 461–2
Zhang Z, Nadeau P, Song W, et al. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat Cell Biol 2000; 2(7): 463–5
Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995; 375(6534): 754–60
Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995; 269(5226): 973–7
Rogaev EI, Sherrington R, Rogaeva EA, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 1995; 376(6543): 775–8
De Strooper B, Beullens M, Contreras B, et al. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J Biol Chem 1997 Feb 7; 272(6): 3590–8
Doan A, Thinakaran G, Borchelt DR, et al. Protein topology of presenilin1. Neuron 1996 Nov; 17(5): 1023–30
Li X, Greenwald I. Membrane topology of the C. elegans SEL-12 presenilin. Neuron 1996 Nov; 17(5): 1015–21
Lehmann S, Chiesa R, Harris DA. Evidence for a six-transmembrane domain structure of presenilin1. J Biol Chem 1997 May 2; 272(18): 12047–51
Nakai T, Yamasaki A, Sakaguchi M, et al. Membrane topology of Alzheimer’s disease-related presenilin 1: evidence for the existence of a molecular species with a seven membrane-spanning and one membrane-embedded structure. J Biol Chem 1999 Aug 13; 274(33): 23647–58
Li X, Greenwald I. Additional evidence for an eight-transmembrane-domain topology for Caenorhabditis elegans and human presenilins. Proc Natl Acad Sci U S A 1998 Jun 9; 95(12): 7109–14
Thinakaran G, Borchelt DR, Lee MK, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 1996; 17(1): 181–90
Henricson A, Kall L, Sonnhammer EL. A novel transmembrane topology of presenilin based on reconciling experimental and computational evidence. FEBS J 2005 Jun; 272(11): 2727–33
Oh YS, Turner RJ. Topology of the C-terminal fragment of human presenilin1. Biochemistry 2005 Sep 6; 44(35): 11821–8
Laudon H, Hansson EM, Melen K, et al. A nine-transmembrane domain topology for presenilin1. J Biol Chem 2005 Oct 21; 280(42): 35352–60
Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid b-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996; 2(8): 864–70
Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid β-protein in both transfected cells and transgenic mice. Nat Med 1997; 3(1): 67–72
Weidemann A, Paliga K, Durrwang U, et al. Formation of stable complexes between two Alzheimer’s disease gene products: presenilin-2 and β-amyloid precursor protein. Nat Med 1997 Mar; 3(3): 328–32
Xia W, Ray WJ, Ostaszewski BL, et al. Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid β-protein generation. Proc Natl Acad Sci U S A 2000; 97(16): 9299–304
Wolfe MS, Xia W, Ostaszewski BL, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 1999; 398(6727): 513–7
Wolfe MS, Xia W, Moore CL, et al. Peptidomimetic probes and molecular modeling suggest that Alzheimer’s γ-secretase is an intramembrane-cleaving aspartyl protease. Biochemistry 1999; 38: 4720–7
Li YM, Xu M, Lai MT, et al. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin1. Nature 2000; 405(6787): 689–94
Esler WP, Kimberly WT, Ostaszewski BL, et al. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat Cell Biol 2000; 2(7): 428–34
Seiffert D, Bradley JD, Rominger CM, et al. Presenilin-1 and -2 are molecular targets for γ-secretase inhibitors. J Biol Chem 2000; 275(44): 34086–91
Evin G, Sharpies RA, Weidemann A, et al. Aspartyl protease inhibitor pepstatin binds to the presenilins of Alzheimer’s disease. Biochemistry 2001; 40(28): 8359–68
LaPointe CF, Taylor RK. The type 4 prepilin peptidases comprise a novel family of aspartic acid proteases. J Biol Chem 2000; 275(2): 1502–10
Steiner H, Kostka M, Romig H, et al. Glycine 384 is required for presenilin-1 function and is conserved in bacterial polytopic aspartyl proteases. Nat Cell Biol 2000; 2(11): 848–51
Weihofen A, Binns K, Lemberg MK, et al. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 2002; 296(5576): 2215–8
McCarthy JV. Involvement of presenilins in cell-survival signalling pathways. Biochem Soc Trans 2005 Aug; 33 (Pt 4): 568–72
Kang DE, Soriano S, Xia X, et al. Presenilin couples the paired phosphorylation of β-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell 2002 Sep 20; 110(6): 751–62
Esselens C, Oorschot V, Baert V, et al. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J Cell Biol 2004 Sep 27; 166(7): 1041–54
Yu G, Chen F, Levesque G, et al. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains β-catenin. J Biol Chem 1998; 273(26): 16470–5
Yu G, Nishimura M, Arawaka S, et al. Nicastrin modulates presenilin-mediated Notch/GLP-1 signal transduction and βAPP processing. Nature 2000; 407(6800): 48–54
Leem JY, Vijayan S, Han P, et al. Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J Biol Chem 2002; 277(21): 19236–40
Edbauer D, Winkler E, Haass C, et al. Presenilin and nicastrin regulate each other and determine amyloid β-peptide production via complex formation. Proc Natl Acad Sci U S A 2002; 99(13): 8666–71
Kimberly WT, LaVoie MJ, Ostaszewski BL, et al. Complex N-linked glycosylated Nicastrin associates with active γ-secretase and undergoes tight cellular regulation. J Biol Chem 2002; 277(38): 35113–7
Yang DS, Tandon A, Chen F, et al. Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J Biol Chem 2002; 277(31): 28135–42
Li T, Ma G, Cai H, et al. Nicastrin is required for assembly of presenilin/γ-secretase complexes to mediate Notch signaling and for processing and trafficking of β-amyloid precursor protein in mammals. J Neurosci 2003 Apr 15; 23(8): 3272–7
Shah S, Lee SF, Tabuchi K, et al. Nicastrin functions as a γ-secretase-substrate receptor. Cell 2005 Aug 12; 122(3): 435–47
Wong PC, Zheng H, Chen H, et al. Presenilin 1 is required for Notchl and DII1 expression in the paraxial mesoderm. Nature 1997; 387(6630): 288–92
Hartmann D, Tournoy J, Saftig P, et al. Implication of APP secretases in Notch signalling. J Mol Neurosci 2001 Oct; 17(2): 171 -181
Lai EC. Notch signaling: control of cell communication and cell fate. Development 2004; 131(5): 965–73
Logeat F, Bessia C, Brou C, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A 1998 Jul 7; 95(14): 8108–12
Kopan R, Schroeter EH, Weintraub H, et al. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci U S A 1996; 93(4): 1683–8
Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 2000; 5(2): 207–16
De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 1999 Apr 8; 398(6727): 518–22
Okochi M, Steiner H, Fukumori A, et al. Presenilins mediate a dual intramembranous γ-secretase cleavage of Notch-1. EMBO J 2002 Oct 15; 21(20): 5408–16
Mizutani T, Taniguchi Y, Aoki T, et al. Conservation of the biochemical mechanisms of signal transduction among mammalian Notch family members. Proc Natl Acad Sci U S A 2001; 98(16): 9026–31
Baumeister R, Leimer U, Zweckbronner I, et al. Human presenilin-1, but not familial Alzheimer’s disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct 1997; 1(2): 149–59
Guo Y, Livne-Bar I, Zhou L, et al. Drosophila presenilin is required for neuronal differentiation and affects Notch subcellular localization and signaling. J Neurosci 1999; 19(19): 8435–42
Wittenburg N, Eimer S, Lakowski B, et al. Presenilin is required for proper morphology and function of neurons in C. elegans. Nature 2000; 406(6793): 306–9
Francis R, McGrath G, Zhang J, et al. APH-1 and PEN-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev Cell 2002; 3(1): 85–97
Goutte C, Tsunozaki M, Hale VA, et al. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A 2002; 99(2): 775–9
Takasugi N, Tomita T, Hayashi I, et al. The role of presenilin cofactors in the γ-secretase complex. Nature 2003; 422(6930): 438–41
Edbauer D, Winkler E, Regula JT, et al. Reconstitution of γ-secretase activity. Nat Cell Biol 2003; 5(5): 486–8
Hayashi I, Urano Y, Fukuda R, et al. Selective reconstitution and recovery of functional γ-secretase complex on budded baculovirus particles. J Biol Chem 2004; 279(36): 38040–6
Steiner H, Winkler E, Edbauer D, et al. PEN-2 is an integral component of the γ-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem 2002; 277(42): 39062–5
Lee SF, Shah S, Li H, et al. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-β precursor protein and Notch. J Biol Chem 2002; 277(47): 45013–9
LaVoie MJ, Fraering PC, Ostaszewski BL, et al. Assembly of the γ-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J Biol Chem 2003; 278(39): 37213–22
Hu Y, Fortini ME. Different cofactor activities in γ-secretase assembly: evidence for a nicastrin-Aph-1 subcomplex. J Cell Biol 2003; 161(4): 685–90
Gu Y, Chen F, Sanjo N, et al. APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin-nicastrin complexes. J Biol Chem 2003; 278(9): 7374–80
Niimura M, Isoo N, Takasugi N, et al. Aph-1 contributes to the stabilization and trafficking of the γ-secretase complex through mechanisms involving intermolecular and intramolecular interactions. J Biol Chem 2005 Apr 1; 280(13): 12967–75
Capell A, Beher D, Prokop S, et al. γ-Secretase complex assembly within the early secretory pathway. J Biol Chem 2005 Feb 25; 280(8): 6471–8
Shirotani K, Edbauer D, Prokop S, et al. Identification of distinct γ-secretase complexes with different APH-1 variants. J Biol Chem 2004 Oct 1; 279(40): 41340–5
Ma G, Li T, Price DL, et al. APH-1a is the principal mammalian APH-1 isoform present in gamma-secretase complexes during embryonic development. J Neurosci 2005 Jan 5; 25(1): 192–8
Serneels L, Dejaegere T, Craessaerts K, et al. Differential contribution of the three Aph1 genes to γ-secretase activity in vivo. Proc Natl Acad Sci U S A 2005 Feb 1; 102(5): 1719–24
Culvenor JG, Ilaya NT, Ryan MT, et al. Characterization of presenilin complexes from mouse and human brain using blue native gel electrophoresis reveals high expression in embryonic brain and minimal change in complex mobility with pathogenic presenilin mutations. Eur J Biochem 2004; 271(2): 375–85
Kimberly WT, LaVoie MJ, Ostaszewski BL, et al. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A 2003; 100(11): 6382–7
Farmery MR, Tjernberg LO, Pursglove SE, et al. Partial purification and characterization of γ-secretase from post-mortem human brain. J Biol Chem 2003; 278(27): 24277–84
Esler WP, Kimberly WT, Ostaszewski BL, et al. Activity-dependent isolation of the presenilin-γ-secretase complex reveals nicastrin and a γ substrate. Proc Natl Acad Sci U S A 2002 Mar 5; 99(5): 2720–5
Fraering PC, LaVoie MJ, Ye W, et al. Detergent-dependent dissociation of active γ-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry 2004; 43(2): 323–33
Fraering PC, Ye W, Strub JM, et al. Purification and characterization of the human γ-secretase complex. Biochemistry 2004; 43(30): 9774–89
Beher D, Fricker M, Nadin A, et al. In vitro characterization of the presenilin-dependent γ-secretase complex using a novel affinity ligand. Biochemistry 2003; 42(27): 8133–42
Gu Y, Sanjo N, Chen F, et al. The presenilin proteins are components of multiple membrane-bound complexes that have different biological activities. J Biol Chem 2004; 279(30): 31329–36
Evin G, Canterford LD, Hoke DE, et al. Transition-state analogue γ-secretase inhibitors stabilize a 900 kDa presenilin/nicastrin complex. Biochemistry 2005 Mar 22; 44(11): 4332–41
Hoke DE, Tan JL, Ilaya NT, et al. In vitro γ-secretase cleavage of the Alzheimer’s amyloid precursor protein correlates to a subset of presenilin complexes and is inhibited by zinc. FEBS J 2005; 272(21): 5544–57
Berezovska O, Ramdya P, Skoch J, et al. Amyloid precursor protein associates with a nicastrin-dependent docking site on the presenilin 1-γ-secretase complex in cells demonstrated by fluorescence lifetime imaging. J Neurosci 2003; 23(11): 4560–6
Ramdya P, Skoch J, Bacskai BJ, et al. Activated Notch1 associates with a presenilin-1/γ-secretase docking site. J Neurochem 2003; 87(4): 843–50
Kornilova AY, Das C, Wolfe MS. Differential effects of inhibitors on the γ-secretase complex. Mechanistic implications. J Biol Chem 2003; 278(19): 16470–3
Saura CA, Tomita T, Davenport F, et al. Evidence that intramolecular associations between presenilin domains are obligatory for endoproteolytic processing. J Biol Chem 1999 May 14; 274(20): 13818–23
Chen F, Tandon A, Sanjo N, et al. Presenilin 1 and presenilin 2 have differential effects on the stability and maturation of nicastrin in mammalian brain. J Biol Chem 2003 May 30; 278(22): 19974–9
Lai MT, Chen E, Crouthamel MC, et al. Presenilin-1 and presenilin-2 exhibit distinct yet overlapping γ-secretase activities. J Biol Chem 2003 Jun 20; 278(25): 22475–81
Mastrangelo P, Mathews PM, Chishti MA, et al. Dissociated phenotypes in presenilin transgenic mice define functionally distinct γ-secretases. Proc Natl Acad Sci U S A 2005 Jun 21; 102(25): 8972–7
Wilson CA, Doms RW, Zheng H, et al. Presenilins are not required for Aβ42 production in the early secretory pathway. Nat Neurosci 2002; 5(9): 849–55
Armogida M, Petit A, Vincent B, et al. Endogenous β-amyloid production in presenilin-deficient embryonic mouse fibroblasts. Nat Cell Biol 2001; 3(11): 1030–3
Selkoe D, Kopan R. Notch and presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 2003; 26: 565–97
Leissring MA, Murphy MP, Mead TR, et al. A physiologic signaling role for the γ-secretase-derived intracellular fragment of APP. Proc Natl Acad Sci U S A 2002; 99(7): 4697–702
von Rotz RC, Kohli BM, Bosset J, et al. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci 2004; 117 (Pt 19): 4435–48
Fortini ME. γ-Secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol 2002; 3(9): 673–84
Eggert S, Paliga K, Soba P, et al. The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves α-, β-, γ-, and ε-like cleavages: modulation of APLP-1 processing by N-glycosylation. J Biol Chem 2004; 279(18): 18146–56
Walsh DM, Fadeeva JV, LaVoie MJ, et al. γ-Secretase cleavage and binding to FE65 regulate the nuclear translocation of the intracellular C-terminal domain (ICD) of the APP family of proteins. Biochemistry 2003; 42(22): 6664–73
Kopan R, Goate A. A common enzyme connects Notch signaling and Alzheimer’s disease. Genes Dev 2000; 14(22): 2799–806
Saxena MT, Schroeter EH, Mumm JS, et al. Murine Notch homologs (N1-4) undergo presenilin-dependent proteolysis. J Biol Chem 2001; 276(43): 40268–73
LaVoie MJ, Selkoe DJ. The Notch ligands, jagged and delta, are sequentially processed by α-secretase and presenilin/γ-secretase and release signaling fragments. J Biol Chem 2003; 278(36): 34427–37
Ikeuchi T, Sisodia SS. The Notch ligands, delta1 and jagged2, are substrates for presenilin-dependent “γ-secretase” cleavage. J Biol Chem 2003; 278(10): 7751–4
Marambaud P, Shioi J, Serban G, et al. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J 2002; 21(8): 1948–56
Marambaud P, Wen PH, Dutt A, et al. A CBP binding transcriptional repressor produced by the PS 1/ε-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 2003; 114(5): 635–45
Haas IG, Frank M, Veron N, et al. Presenilin-dependent processing and nuclear function of gamma-protocadherins. J Biol Chem 2005 Mar 11; 280(10): 9313–9
Lammich S, Okochi M, Takeda M, et al. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Aβ-like peptide. J Biol Chem 2002; 277(47): 44754–9
Murakami D, Okamoto I, Nagano O, et al. Presenilin-dependent γ-secretase activity mediates the intramembranous cleavage of CD 44. Oncogene 2003; 22(10): 1511–6
Andersson CX, Fernandez-Rodriguez J, Laos S, et al. Shedding and γ-secretase mediated intramembrane proteolysis of the mucin-type molecule CD 43. Biochem J 2005 Apr 15; 387 (Pt 2): 377–84
May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem 2002; 277(21): 18736–43
Meyer EL, Strutz N, Gahring LC, et al. Glutamate receptor subunit 3 is modified by site-specific limited proteolysis including cleavage by γ-secretase. J Biol Chem 2003; 278(26): 23786–96
McCarthy JV. Involvement of presenilins in cell-survival signalling pathways. Biochem Soc Trans 2005; 33(4): 568–72
Marambaud P, Robakis NK. Genetic and molecular aspects of Alzheimer’s disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav 2005 Apr; 4(3): 134–46
Saura CA, Choi SY, Beglopoulos V, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 2004 Apr 8; 42(1): 23–36
Saura CA, Chen G, Malkani S, et al. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J Neurosci 2005 Jul 20; 25(29): 6755–64
Coolen MW, Van Loo KM, Van Bakel NN, et al. Gene dosage effect on γ-secretase component Aph-1b in a rat model for neurodevelopmental disorders. Neuron 2005 Feb 17; 45(4): 497–503
Lee HJ, Jung KM, Huang YZ, et al. Presenilin-dependent γ-secretase-like intramembrane cleavage of ErbB 4. J Biol Chem 2001; 277(8): 6318–23
Ni CY, Murphy MP, Golde TE, et al. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 2001; 294(5549): 2179–81
Kim DY, MacKenzie Ingano LA, Kovacs DM. Nectin-1α, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/γ-secretase-like cleavage. J Biol Chem 2002; 277(51): 49976–81
Schulz JG, Annaert W, Vandekerckhove J. Syndecan 3 intramembrane proteolysis is presenilin/γ-secretase-dependent and modulates cytosolic signaling. J Biol Chem 2003; 278(49): 48651–7
Maretzky T, Schulte M, Ludwig A, et al. Ll is sequentially processed by two differently activated metalloproteases and presenilin/γ-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol 2005 Oct; 25(20): 9040–53
Kanning KC, Hudson M, Amieux PS, et al. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci 2003; 23(13): 5425–36
Gowrishankar K, Zeidler MG, Vincenz C. Release of a membrane-bound death domain by a-secretase processing of the p75NTR homolog NRADD. J Cell Sci 2004; 117 (Pt 18): 4099–111
Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “γ-secretase” processing of deleted in colorectal cancer (DCC). J Biol Chem 2003; 278(33): 30425–8
Kim DY, Ingano LA, Carey BW, et al. Presenilin/γ-secretase-mediated cleavage of the voltage-gated sodium channel β2-subunit regulates cell adhesion and migration. J Biol Chem 2005 Jun 17; 280(24): 23251–61
Cowan JW, Wang X, Guan R, et al. Growth hormone receptor is a target for presenilin-dependent gamma-secretase cleavage. J Biol Chem 2005 May 13; 280(19): 19331–42
Wang R, Tang P, Wang P, et al. Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer’s disease mutation. Proc Natl Acad Sci U S A 2006 Jan 10; 103(2) 353–8
Fuller SJ, Storey E, Li QX, et al. Intracellular production of βA4 amyloid of Alzheimer’s disease: modulation by phosphoramidon and lack of coupling to the secretion of the amyloid precursor protein. Biochemistry 1995; 34(25): 8091–8
Higaki J, Quon D, Zhong Z, et al. Inhibition of β-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron 1995; 14(3): 651–9
Sernee MF, Evin G, Culvenor JG, et al. Selecting cells with different Alzheimer’s disease γ-secretase activity using FACS: differential effect on presenilin exon 9 γ- and ε-cleavage. Eur J Biochem 2003; 270(3): 495–506
Cao X, Sudhof TC. A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip 60. Science 2001; 293(5527): 115–20
Komano H, Shiraishi H, Kawamura Y, et al. A new functional screening system for identification of regulators for the generation of amyloid β-protein. J Biol Chem 2002; 277(42): 39627–33
Karlstrom H, Bergman A, Lendahl U, et al. A sensitive and quantitative assay for measuring cleavage of presenilin substrates. J Biol Chem 2002; 277(9): 6763–6
Liao YF, Wang BJ, Cheng HT, et al. Tumor necrosis factor-α, interleukin-1 β, and interferon-γ stimulate γ-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem 2004; 279(47): 49523–32
Steiner H, Pesold B, Haass C. An in vivo assay for the identification of target proteases which cleave membrane-associated substrates. FEBS Lett 1999; 463(3): 245–9
McLendon C, Xin T, Ziani-Cherif C, et al. Cell-free assays for γ-secretase activity. FASEB J 2000; 14(15): 2383–6
Ikeuchi T, Sisodia SS. Cell-free generation of the Notch1 intracellular domain (NICD) and APP-CTFγ. evidence for distinct intramembranous “γ-secretase” activities. Neuromolecular Med 2002; 1(1): 43–54
Pinnix I, Musunuru U, Tun H, et al. A novel γ-secretase assay based on detection of the putative C-terminal fragment-γ of amyloid β protein precursor. J Biol Chem 2001; 276(1): 481–7
Kimberly WT, Esler WP, Ye W, et al. Notch and the amyloid precursor protein are cleaved by similar γ-secretase (s). Biochemistry 2003; 42(1): 137–44
Wrigley JD, Schurov I, Nunn EJ, et al. Functional overexpression of γ-secretase reveals protease-independent trafficking functions and a critical role of lipids for protease activity. J Biol Chem 2005 Apr 1; 280(13): 12523–35
Ogura T, Mio K, Hayashi I, et al. Three-dimensional structure of the gamma-secretase complex. Biochem Biophys Res Commun 2006 May 5; 343(2): 525–34
Klafki H, Abramowski D, Swoboda R, et al. The carboxyl termini of β-amyloid peptides 1-40 and 1-42 are generated by distinct γ-secretase activities. J Biol Chem 1996; 271(45): 28655–9
Esler WP, Kimberly WT, Ostaszewski BL, et al. Activity-dependent isolation of the presenilin-γ-secretase complex reveals nicastrin and a y substrate. Proc Natl Acad Sci U S A 2002; 99(5): 2720–5
Kornilova AY, Das C, Wolfe MS. Differential effects of inhibitors on the γ-secretase complex: mechanistic implications. J Biol Chem 2003; 278(19): 16470–3
Piper SC, Amtul Z, Galinanes-Garcia L, et al. Peptide-based, irreversible inhibitors of γ-secretase activity. Biochem Biophys Res Commun 2003; 305(3): 529–33
Bihel F, Das C, Bowman MJ, et al. Discovery of a Subnanomolar helical D-tridecapeptide inhibitor of γ-secretase. J Med Chem 2004; 47(16): 3931–3
Kornilova AY, Bihel F, Das C, et al. The initial substrate-binding site of γ-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A 2005 Mar 1; 102(9): 3230–5
Bunnell WL, Pham HV, Glabe CG. γ-Secretase cleavage is distinct from endoplasmic reticulum degradation of the trans-membrane domain of the amyloid precursor protein. J Biol Chem 1998; 273(48): 31947–55
Skovronsky DM, Pijak DS, Doms RW, et al. A distinct ER/IC γ-secretase competes with the proteasome for cleavage of APP. Biochemistry 2000; 39(4): 810–7
Lewis HD, Perez Revueita BI, Nadin A, et al. Catalytic site-directed γ-secretase complex inhibitors do not discriminate pharmacologically between Notch S3 and β-APP cleavages. Biochemistry 2003; 42(24): 7580–6
Higaki JN, Chakravarty S, Bryant CM, et al. A combinatorial approach to the identification of dipeptide aldehyde inhibitors of β-amyloid production. J Med Chem 1999; 42(19): 3889–98
Citron M, Diehl TS, Gordon G, et al. Evidence that the 42- and 40-amino acid forms of amyloid β protein are generated from the β-amyloid precursor protein by different protease activities. Proc Natl Acad Sci U S A 1996; 93(23): 13170–5
Figueiredo-Pereira ME, Efthimiopoulos S, Tezapsidis N, et al. Distinct secretases, a cysteine protease and a serine protease, generate the C-termini of amyloid β-proteins Aβ1-40 and Aβ1-42, respectively. J Neurochem 1999; 72: 1417–22
Tian G, Sobotka-Briner CD, Zysk J, et al. Linear non-competitive inhibition of solubilized human γ-secretase by pepstatin A methylester, L-685458, sulfonamides and benzodiazepines. J Biol Chem 2002; 277(35): 31499–505
Dovey HF, John V, Anderson JP, et al. Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J Neurochem 2001; 76(1): 173–81
Comery TA, Martone RL, Aschmies S, et al. Acute γ-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci 2005 Sep 28; 25(39): 8898–902
Micchelli CA, Esler WP, Kimberly WT, et al. γ-Secretase/presenilin inhibitors for Alzheimer’s disease phenocopy Notch mutations in Drosophila. FASEB J 2003; 17(1): 79–81
Geling A, Steiner H, Willem M, et al. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 2002; 3(7): 688–94
Petit A, Bihel F, Alves da Costa C, et al. New protease inhibitors prevent γ-secretase-mediated production of Aβ40/42 without affecting Notch cleavage. Nat Cell Biol 2001; 3(5): 507–11
Petit A, Pasini A, Alves da Costa C, et al. JLK isocoumarin inhibitors: selective γ-secretase inhibitors that do not interfere with Notch pathway in vitro or in vivo. J Neurosci Res 2003; 74(3): 370–7
Petit A, Dumanchin-Njock C, Andrau D, et al. Amyloid-lowering isocoumarins are not direct inhibitors of γ-secretase. Nat Cell Biol 2002; 4(5): E111–2
Esler WP, Das C, Campbell WA, et al. Amyloid-lowering isocoumarins are not direct inhibitors of γ-secretase. Nat Cell Biol 2002; 4(5): E110–1
Bihel F, Quelever G, Lelouard H, et al. Synthesis of new 3-alkoxy-7-amino-4-chloro-isocoumarin derivatives as new β-amyloid peptide production inhibitors and their activities on various classes of protease. Bioorg Med Chem 2003; 11(14): 3141–52
Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 2004; 279(13): 12876–82
Lanz TA, Hosley JD, Adams WJ, et al. Studies of Aβ pharmacodynamics in the brain, cerebrospinal fluid, and plasma in young (plaque-free) Tg2576 mice using the γ-secretase inhibitor N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo -6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide (LY-411575). J Pharmacol Exp Ther 2004; 309(1): 49–55
Barten DM, Guss VL, Corsa JA, et al. Dynamics of β-amyloid reductions in brain, cerebrospinal fluid and plasma of β-amyloid precursor protein transgenic mice treated with a γ-secretase inhibitor. J Pharmacol Exp Ther 2005 Feb; 312(2): 635–43
Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 2001; 414(6860): 212–616
Takahashi Y, Hayashi I, Tominari Y, et al. Sulindac sulfide is a non-competitive γ-secretase inhibitor that preferentially reduces Aβ42 generation. J Biol Chem 2003; 278(20): 18664–70
Morihara T, Chu T, Ubeda O, et al. Selective inhibition of Aβ42 production by NSAID R-enantiomers. J Neurochem 2002; 83(4): 1009–12
Beher D, Clarke EE, Wrigley JD, et al. Selected non-steroidal anti-inflammatory drugs and their derivatives target γ-secretase at a novel site: evidence for an allosteric mechanism. J Biol Chem 2004; 279(42): 43419–26
Weggen S, Eriksen JL, Sagi SA, et al. Aβ42-lowering nonsteroidal anti-inflammatory drugs preserve intramembrane cleavage of the amyloid precursor protein (APP) and ErbB-4 receptor and signaling through the APP intracellular domain. J Biol Chem 2003; 278(33): 30748–54
Harrison T, Churcher I, Beher D. γ-Secretase as a target for drug intervention in Alzheimer’s disease. Curr Opin Drug Discov Devel 2004; 7(5): 709–19
Lanz TA, Himes CS, Pallante G, et al. The γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces A β levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. J Pharmacol Exp Ther 2003; 305(3): 864–71
Lleo A, Berezovska O, Herl L, et al. Nonsteroidal anti-inflammatory drugs lower Aβ42 and change presenilin 1 conformation. Nat Med 2004; 10(10): 1065–6
van Es JH, van Gijn ME, Riccio O, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005 Jun 16; 435(7044): 959–63
Minter LM, Turley DM, Das P, et al. Inhibitors of γ-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx 21. Nat Immunol 2005 Jul; 6(7): 680–8
A Notch signalling pathway inhibitor for patients with advanced breast cancer (sponsored by Merck) [online]. Available from URL: http://www.clinicaltrials.gov/ct/show/NCT00106145 [Accessed 2006 Apr 11]
A Notch signalling pathway inhibitor for patients with T-cell acute lymphoblastic leukemia/lymphoma (sponsored by Merck) [online]. Available from URL: http://www.clinicaltrials.gov/ct/show/NCT00100152 [Accessed 2006 Apr 11]
Beher D, Wrigley JD, Nadin A, et al. Pharmacological knockdown of the presenilin 1 heterodimer by a novel γ-secretase inhibitor: implications for presenilin biology. J Biol Chem 2001; 276(48): 45394–402
Campbell WA, Reed ML, Strahle J, et al. Presenilin endoproteolysis mediated by an aspartyl protease activity pharmacologically distinct from γ-secretase. J Neurochem 2003; 85(6): 1563–74
Tarassishin L, Yin YI, Bassit B, et al. Processing of Notch and amyloid precursor protein by γ-secretase is spatially distinct. Proc Natl Acad Sci U S A 2004 Dec 7; 101(49): 17050–5
Espeseth AS, Xu M, Huang Q, et al. Compounds that bind APP and inhibit Aβ processing in vitro suggest a novel approach to Alzheimer disease therapeutics. J Biol Chem 2005 May 6; 280(18): 17792–7
Phiel CJ, Wilson CA, Lee VM, et al. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 2003; 423(6938): 435–9
Sai X, Kawamura Y, Kokame K, et al. Endoplasmic reticulum stress-inducible protein, Herp, enhances presenilin-mediated generation of amyloid β-protein. J Biol Chem 2002; 277(15): 12915–20
Netzer WJ, Dou F, Cai D, et al. Gleevec inhibits β-amyloid production but not Notch cleavage. Proc Natl Acad Sci U S A 2003; 100(21): 12444–9
Fraering PC, Ye W, Lavoie MJ, et al. γ-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide binding site. J Biol Chem 2005 Dec 23; 280(51): 41987–96
Siemers E, Skinner M, Dean RA, et al. Safety, tolerability and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol 2005 May–Jun; 28(3): 126–32
Siemers ER, Quinn JF, Kaye J. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology 2006 Feb 28; 66(4): 602–4
Acknowledgements
Geneviève Evin and Colin L. Masters are supported by the National Health and Medical Research Council (Australia), grants 114150 and 400073. Colin L. Masters is Executive Director and Chairman of the Scientific Advisory Board of Prana Biotechnology Ltd, a company aimed at providing therapeutic intervention for age-related disease. All authors declare they have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evin, G., Sernee, M.F. & Masters, C.L. Inhibition of γ-Secretase as a Therapeutic Intervention for Alzheimer’s Disease. CNS Drugs 20, 351–372 (2006). https://doi.org/10.2165/00023210-200620050-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200620050-00002