Abstract
Objective: To collect descriptive data on the treatment of Alzheimer’s disease with galantamine under naturalistic conditions.
Study design: This was a prospective, open-label, observational study.
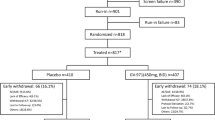
Patients: Subjects (n = 345) with mild to moderately severe dementia of the Alzheimer’s type were recruited from 48 hospitals in Australia.
Methods: Subjects were enrolled and received treatment with galantamine for 6 months in a clinical practice setting. Subjects were assessed at baseline and 3 and 6 months after starting treatment using the Mini-Mental State Examination (MMSE), the Clinician’s Interview-Based Impression of Change plus Caregiver Input (CIBIC-plus) and the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) [the latter only if the baseline MMSE score was at least 25]. Subjects were also assessed using an abridged Instrumental Activities of Daily Living (IADL) questionnaire that included questions on using the telephone, ability to travel more than 1km outside the home, taking medications and managing money, and an 11-item behaviour assessment scale that measured aggression, sleep disturbance, disinhibition, personality changes, irritability, depression, agitation, apathy, inertia, hallucinations and aberrant motor behaviour.
Results: Of the 345 subjects who were enrolled in the study (intent-to-treat [ITT] population), 229 completed the baseline, 3- and 6-month visits (per-protocol [PP] population). The mean age of the PP population was 78.0 ± 6.8 years.
At 6 months, most PP subjects (70%) showed an increase in MMSE score compared with baseline, with a mean increase in score of 2.0 ± 3.1 points from a baseline of 20.8 ± 4.2 points. In the ITT population, 44% of subjects (151/345) showed an increase in MMSE after 6 months. If data were unavailable the patient was classified as a nonresponder. Of the 21 PP patients who were assessed using ADAS-cog, 18 (86%) demonstrated a decrease in the ADAS-cog score, reflecting an improvement in cognition. Of the ITT population, 33% (19/57) had a decreased ADAS-cog score after 6 months.
Most PP subjects (86%) were considered responders according to the CIBIC-plus score, with 65% showing some improvement over 6 months of treatment. Of the ITT population, 54% (187/345) showed no deterioration in CIBIC-plus score after 6 months.
No deterioration in IADL or behaviour assessments occurred in the majority of PP subjects over 6 months.
Conclusions: In a clinical practice setting, the majority of subjects receiving galantamine who completed the study maintained their ratings of cognition, function, behaviour or global assessment over the 6-month period.






Similar content being viewed by others
Notes
1 Reminyl is the registered trademark of Janssen-Cilag Pty Ltd, Australia, for galantamine preparations. The use of trade names is for product identification purposes only and does not imply endorsement.
2 Positive changes in MMSE scores represent clinical improvement whereas negative changes in ADAS-cog scores represent clinical improvement.
References
Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366: 2112–7
Access Economics Pty Ltd. Dementia estimates and projections: Australian states and territories (February 2005) [online]. Available from URL: http://www.alzheimers.org.au/upload/Access%20Report%20Feb%202005.pdf [Accessed 2005 Jun 16]
Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 25: CD005593
Scott L, Goa K. Galantamine: a review of its use in Alzheimer’s disease. Drugs 2000; 60: 1095–122
Australian Government, Department of Health & Ageing. Schedule of pharmaceutical benefits for approved pharmacists and medical practitioners [online]. Available from URL: http://www.health.gov.au/pbs [Accessed 2005 May 31]
Folstein M, Folstein S, McHugh P. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98
Rosen W, Mohs R, Davis K. A new rating scale for Alzheimer’s disease. Am J Psychiatr 1984; 141: 1356–64
Blesa R. Galantamine: therapeutic effects beyond cognition. Dement Geriatr Cogn Disord 2000; 11Suppl. 1: 28–34
Bores G, Huger F, Petko W, et al. Pharmacological evaluation of novel Alzheimer’s disease therapeutics: acetylcholinesterase inhibitors related to galantamine. J Pharmacol Exp Ther 1996; 277: 728–38
Tariot P, Solomon P, Morris J, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 2000; 54: 2269–76
Raskind M, Peskind E, Wessel T, et al. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000; 54: 2261–8
Cummings J, Schneider L, Tariot P, et al. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry 2004; 161: 532–8
Therapeutic Goods Administration. Australian-approved product information for galantamine (REMINYL®). Canberra: Department of Health & Ageing; Approved 2005 Mar 22
Schneider L, Olin J, Doody R, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study: Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11 Suppl. 2: S22–32
Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86
Australian Government, Department of Health & Ageing, Therapeutic Goods Administration. Australian Adverse Drug Reactions Advisory Committee (ADRAC) [online]. Available from URL: http://www.tga.gov.au/adr/adrfaq.htm [Accessed 2005 Jun 16]
Patterson C, Passmore A, Crawford V. A 6-month open-label study of the effectiveness and tolerability of galantamine in patients with Alzheimer’s disease. Int J Clin Pract 2004; 58: 144–8
Lopez-Pousa S, Turon-Estrada A, Garre-Olmo J, et al. Differential efficacy of treatment with acetylcholinesterase inhibitors in patients with mild and moderate Alzheimer’s disease over a 6-month period. Dement Geriatr Cogn Disord 2005; 19: 189–95
Acknowledgements
The authors thank the Australian clinicians and the subjects and caregivers who participated in this study. They also acknowledge Quintiles Health Research Solutions for data management and statistical support, and Peter Tobin (Medical Research, Janssen-Cilag Australia) for assistance with preparation of the manuscript.
This study was conducted with financial assistance from Janssen-Cilag Pty Ltd, Australia. Professor Henry Brodaty, Associate Professor Michael Woodward and Dr Karyn Boundy are consultants, supported speakers and supported investigators for Janssen-Cilag Australia. Gabrielle Allen and Nicola Barnes are employed by Janssen-Cilag Australia, and own stock in the company
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brodaty, H., Woodward, M., Boundy, K. et al. A Naturalistic Study of Galantamine for Alzheimer’s Disease. CNS Drugs 20, 935–943 (2006). https://doi.org/10.2165/00023210-200620110-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200620110-00006