Summary
The novel antidepressant mirtazapine is a racemate with both noradrenergic and serotonergic potentiating effects. In vitro metabolism of racemic mirtazapine was studied in (a) microsomes from cells expressing different cytochrome P450 (CYP) enzymes and (b) human liver microsomes from 10 subjects and compared with the rate of metabolism of 4 substrates of specific CYP enzymes: ethoxyresorufin (CYP1A2), diclofenac (CYP2C9), bufuralol (CYP2D6) and testosterone (CYP3A4). These experiments suggested that the 8-hydroxylation of mirtazapine is catalysed by CYP2D6, and that the N(2)-demethylation and N(2)-oxidation are catalysed by CYP3A4. Mirtazapine was shown to be a competitive inhibitor of the CYP2D6 mediated hydroxylation of bufuralol in vitro, but with a 10 times higher inhibition constant (Ki) than for fluoxetine, i.e. mirtazapine is a much weaker inhibitor.
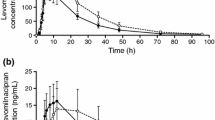
To investigate the importance of CYP2D6 for the in vivo disposition of mirtazapine, a single oral dose of racemic mirtazapine was given to 7 extensive (EM) and 7 poor metabolisers (PM) of debrisoquine. Plasma concentrations (sum of enantiomers) of mirtazapine and its demethyl metabolite were monitored over 3 days. Oral plasma clearance of mirtazapine was very similar in EM and PM of debrisoquine (0.51 ±0.18 and 0.49 ± 0.22 L/h/kg, respectively; NS). In addition, the mean half-lives were almost identical: 23.4 and 23.3 hours, respectively. Plasma concentrations of demethylmirtazapine were also very similar in EM and PM. This in vivo study indicates that the major enantiomer of mirtazapine in plasma is not metabolised by CYP2D6, but it cannot be excluded that the minor one is. A study using a chiral analysis of the 2 enantiomers is currently ongoing.
From a clinical point of view it is unlikely that mirtazapine would inhibit the metabolism of coadministered drugs metabolised by CYP1A2, CYP2D6 or CYP3A4. The panel study on EM and PM shows that strong inhibitors of CYP2D6 would not be expected to affect the concentration of the major mirtazapine enantiomer, but the effect on the minor enantiomer cannot be predicted.
Similar content being viewed by others
References
Davis R, Wilde MI. Mirtazapine: a review of its pharmacology and therapeutic potential in the management of major depression. CNS Drugs 1996; 5: 389–402
De Boer Th, Ruight GSF, Berendsen HHG. The α2-selective adrenoceptor antagonist Org 3770 (mirtazapine, Remeron) enhances noradrenergic and serotonergic neurotransmission. Hum Psychopharmacol 1995; 10 Suppl. 2: S107–S118
Sandker GW, Vos RME, Delbressine LPC, et al. Metabolism of three pharmacologically active drugs in isolated human and rat hepatocytes: analysis of interspecies variability and comparison with metabolism in vivo. Xenobiotica 1994; 24: 143–55
Bertilsson L, Lou YQ, Du YL, et al. Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquine and S-mephenytoin. Clin Pharmacol Ther 1992; 51: 388–97
Bertilsson L, Dahl ML. Polymorphic drug oxidation. Relevance to the treatment of psychiatric disorders. CNS Drugs 1996; 3: 200–23
Kaspersen FM, van Rooij FAM, Sperling EMG, et al. The synthesis of Org 3770 labelled with 3H, 13C and 14C. J Lab Comp Radiopharm 1989; 27: 1055–68
Bogaards JJP, van Ommen B, van Bladeren PJ. Human cytochrome P450 enzyme selectivities in the oxidation of chlorinated benzenes. Toxicol Appl Pharmacol 1995; 132: 44–52
Leemann T, Transon C, Dayer P. Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4′-hydroxylation in human liver. Life Sci 1992; 52: 29–34
Perella FW. EZ-FIT: a practical curve-fitting microcomputer program for the analysis of enzyme kinetic data on IBM-PC compatible computers. Anal Biochem 1988; 174: 437–47
Sanz EJ, Villén T, Alm C, et al. S-mephenytoin hydroxylation phenotype in a Swedish population determined after co-administration with debrisoquin. Clin Pharmacol Ther 1989; 45: 495–9
Mahgoub A, Idle JR, Dring DG, et al. Polymorphic hydroxylation of debrisoquine in man. Lancet 1977; II: 584–6
Lennard MS, Silas JH, Smith AJ, et al. Determination of debrisoquine and its 4-hydroxy metabolite in biological fluids by gas chromatography with flame ionization and nitrogen-selective detection. J Chromatogr 1977; 133: 161–6
Evans DAP, Mahgoub A, Sloan TP, et al. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet 1980; 17: 102–5
Timmer CJ, Lohmann AAM, Mink CPA. Pharmacokinetic dose-proportionality study at steady state of mirtazapine from Remeron® tablets. Hum Psychopharmacol 1995; 10: S97–S106
von Bahr C, Spina E, Birgersson C, et al. Inhibition of desmethylimipramine 2-hydroxylation by drugs in human liver microsomes. Biochem Pharmacol 1985; 34: 2501–5
Inaba T, Jurima M, Mahon WA, et al. In vitro inhibition studies of two isozymes of human liver cytochrome P-450. Drug Metab Dispos 1985; 13: 443–8
Dahl M-L, Tybring G, Elwin CE, et al. Stereoselective disposition of mianserin is related to debrisoquine hydroxylation polymorphism. Clin Pharmacol Ther 1994; 56: 176–83
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dahl, ML., Voortman, G., Alm, C. et al. In Vitro and In Vivo Studies on the Disposition of Mirtazapine in Humans. Clin. Drug Invest. 13, 37–46 (1997). https://doi.org/10.2165/00044011-199713010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199713010-00005