Abstract
Background: The objective of the study was to evaluate the effects of alendronic acid once weekly relative to risedronic acid once weekly on bone mineral density (BMD), markers of bone turnover and tolerability in the treatment of osteoporosis in postmenopausal women.
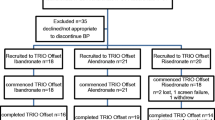
Methods: This was a randomised, double-masked, double-dummy multicentre international study (75 centres in 27 countries in Europe, the Americas and Asia-Pacific). A total of 1303 women were screened and 936 with low bone density (T-score ≤−2.0 at the spine, hip trochanter, total hip or femoral neck) were randomised; 91% (n = 854) completed the study. Patients were randomised to treatment with either active alendronic acid 70mg weekly (Fosamax®) and placebo identical to risedronic acid weekly or active risedronic acid 35mg weekly (Actonel®) and placebo identical to alendronic acid weekly for 12 months. The primary efficacy endpoint was the percentage change from baseline in hip trochanter BMD at 12 months. Secondary endpoints included the percentage change from baseline in lumbar spine, total hip and femoral neck BMD; biochemical markers of bone turnover (including serum bone-specific alkaline phosphatase [BSAP] and urinary type I collagen N-telopeptides [NTx]); and safety and tolerability as assessed by reporting of adverse experiences.
Results: Alendronic acid produced greater increases in BMD than did risedronic acid at 12 months at all sites measured. Mean percentage increases from baseline in hip trochanter BMD at month 12 were 3.56% and 2.71% in the alendronic acid and risedronic acid groups, respectively (treatment difference [95% CI]: 0.83% [0.22, 1.45; p = 0.008]). Mean percentage increases from baseline were greater with alendronic acid than risedronic acid at the lumbar spine, total hip and femoral neck BMD at month 12 (p = 0.002, p < 0.001, p = 0.039, respectively). Increases in BMD with alendronic acid compared with risedronic acid were also significantly greater at 6 months at the trochanter and total hip. There was a greater reduction in bone turnover with alendronic acid compared with risedronic acid: NTx decreased 58% with alendronic acid compared with 47% with risedronic acid at 12 months (p < 0.001); and BSAP decreased 45% with alendronic acid compared with 34% with risedronic acid at 12 months (p < 0.001). Overall tolerability and upper gastrointestinal tolerability were similar for both agents.
Conclusions: Alendronic acid once weekly produced greater BMD increases at both hip and spine sites and greater reductions in bone turnover relative to risedronic acid once weekly. Both agents were well tolerated with no significant difference in upper gastrointestinal adverse experiences. Clinicians should consider these results when making treatment decisions for postmenopausal women with osteoporosis.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
McClung MR. Bisphosphonates in osteoporosis: recent clinical experience. Exp Opin Pharmacother 2000; 1: 225–38
Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 2000; 85: 231–6
Hochberg MC, Greenspan S, Wasnich RD, et al. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 2002; 87: 1586–92
Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with post-menopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42: 1246–54
Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res 2004; 19: 1250–8
Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 2002; 112: 281–9
Li Z, Meredith MP, Hoseyni MS. A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med 2001; 20: 3175–88
Marcus R, Wong M, Heath III H, et al. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev 2002; 23: 16–37
Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 2004; 34: 599–604
Miller PD, Hochberg MC, Wehren LE, et al. How useful are measures of BMD and bone turnover? Curr Med Res Opin 2005; 21: 545–53
Cummings SR, Black DM, Thompson DE, et al. Alendronate reduces the risk of vertebral fractures in women without preexisting vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280: 2077–82
Liberman UA, Weiss SR, Broil J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 1995; 333: 1437–43
Garnero P, Shih WJ, Gineyts E, et al. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab 1994; 79: 1693–700
Bone HG, Hosking D, Devogelaer J-P, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350: 1189–99
Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis: II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 2002; 23: 508–16
Häuselmann HJ, Rizzoli R. A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int 2003; 14: 2–12
Levis S, Quandt SA, Thompson D, et al. Alendronate reduces the risk of multiple symptomatic fractures: results from the Fracture Intervention Trial. J Am Geriatr Soc 2002; 50: 409–15
Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000; 85: 4118–24
Pols HAP, Felsenberg D, Hanley, et al. Multinational, placebo controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int 1999; 9: 461–8
Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 1999; 282: 1344–52
Cranney A, Guyatt G, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis: IX. Summary of meta-analysis of therapies for postmenopausal women. Endocr Rev 2002; 23: 570–7
Kanis JA, Oden A, Johnell O, et al. Uncertain future of trials in osteoporosis. Osteoporos Int 2002; 13: 443–9
McAlister FA, Laupacis A, Wells GA, et al. Users’ guides to the medical literature: XIX. Applying clinical trial results. B. Guidelines for determining whether a drug is exerting (more than) a class effect. JAMA 1999; 282: 1371–7
Bucher HC, Guyatt GH, Cook DJ, et al. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate endpoints: Evidence-Based Medicine Working Group. JAMA 1999; 282: 771–8
Hosking D, Adami S, Felsenberg D, et al. Comparison of change in bone resorption and bone mineral density with once weekly alendronate and daily risedronate. Curr Med Res Opin 2003; 19: 383–94
Rosen C, Hochberg M, Bonnick S, et al. Treatment with once-weekly alendronate 70mg compared to once-weekly risedronate 35mg in women with postmenopausal osteoporosis: a randomized, double-blind study. J Bone Miner Res 2005; 20: 141–51
Khosla S. Surrogates for fracture endpoints in clinical trials. J Bone Miner Res 2003; 18: 1146–9
Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. Lancet 1993; 341: 72–5
Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18: 1051–6
Epstein S. The roles of BMD, turnover, and other properties in reducing fracture risk during antiresorptive therapy. Mayo Clin Proc 2005; 80: 379–88
Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 2004; 7: 255–61
Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom 2001; 4: 105–10
Chestnut III CH, Rosen CJ, for the Bone Quality Discussion Group. Reconsidering the effects of antiresorptive therapies in reducing osteoporotic fracture. J Bone Miner Res 2001; 16: 2163–72
Acknowledgements
This clinical trial was funded by Merck & Co., Inc.; no funding was received for manuscript development. D.M. Reid, D. Hosking, D. Kendler, M.L. Brandi and J.D. Wark have served as paid consultants and/or speakers for Merck & Co., Inc. M.L. Brandi has served as a paid speaker for Procter and Gamble Pharmaceuticals. K. Gaines, N. Verbruggen and M.E. Melton are employees of Merck & Co., Inc. and potentially own stock and/or hold stock options in the company.
The authors would like to recognise the valuable contributions of Christine Sisk to the writing of earlier versions of this manuscript and the expert review and comments to the manuscript provided by Philip Ross, PhD, both of Merck. The authors would also like to recognise the efforts of the study staff at the investigative sites, including the following lead investigators: Australia: J. Eden, J. Graham, M. Hooper, E. Seeman, J. Wark, C. White; Belgium: S. Boonen, Y. Boutsen, J. Kaufman, M. Malaise, J.-Y. Reginster. Brazil: J.F.M. Neto, J.R. Provenza; Canada: J. Brown, D. Kendler, A. Khan; Chile: A. Brizzolara, L. Villanueva; Costa Rica: A. Cob, W. Rios; Dominican Republic: J.V. de Camps, C. Velazco; Ecuador: C. Bracho; Finland: M. Valimaki; France: C.-L. Benhamou, F. Blotman, P. Delmas, L. Euller-Ziegler, P. Fardellone, Y. Maugars, C. Ribot, T. Thomas, G. Weryha; Greece: A. Avramides; Hungary: L. Koranyi, P. Lakatos; Italy: M.L. Brandi, C.E. Fiore, Q. Mela, R. Nuti; Jordan: B. Masri; Latvia: A. Lejnieks, I. Rasa; Lebanon: H. Awada, G. Maalouf, C. Saab; Lithuania: V. Alekna, M. Tamulaitiene, L. Valius; Malaysia: S.P. Chan; Peru: L. Danckers, R. Olavide, R. Salinas; Poland: R. Lorenc, S. Mackiewicz, T. Pertyński; Portugal: A. Faustino, J.M. Gomes; Slovenia: A. Kocijančič; Spain: J.J. Gomez-Reino, C. Lozano-Tonkin, J.L. Perez-Castrillon, M. Rodriguez-Perez, J. Roman-Ivorra; Taiwan: J.-F. Chen, S.-M. Hou; Thailand: B. Ongphiphadhanakul, K. Wilawan; UK: M. Davie, D. Hosking, R. Keen, D. Reid, O. Sahota, P. Selby, M. Stone; Venezuela: T. López.
[ClinicalTrials.gov registry number: 00092040]
Author information
Authors and Affiliations
Corresponding author
Additional information
Fosamax Actonel Comparison Trials
Rights and permissions
About this article
Cite this article
Reid, D.M., Hosking, D., Kendler, D. et al. Alendronic Acid Produces Greater Effects than Risedronic Acid on Bone Density and Turnover in Postmenopausal Women with Osteoporosis. Clin. Drug Investig. 26, 63–74 (2006). https://doi.org/10.2165/00044011-200626020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200626020-00002