Abstract
Introduction
The entomopathogenic nematodes have been reported from all continents (except Antarctica) and almost all regions of the world. Surveys of EPNs in India has resulted in the recovery of several isolates of Steinernema. Among one of them, isolate CS34 was identified as S. hermaphroditum Stock, Griffin & Chaerani, 2004. We investigated the identification and the pathogenicity of S. hermaphroditum in District Meerut of Western Uttar Pradesh, India.
Materials and methods
The Steinernema was examined for its pathogenicity and accurate identification by the mean of morphological and molecular technique and its geographical distribution was mapped based on meta-analysis of the ITS GenBank records.
Results
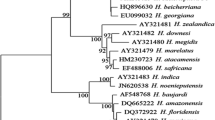
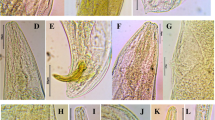
The surveys of agricultural soils of district Meerut, India, resulted in the isolation of one strain from entomopathogenic nematode labelled CS34 through Galleria baiting technique. Morphological characters and morphometrical analysis indicated that the strain CS34 was closely related to the “glaseri” group of Steinernema spp. The Nblast results indicated that ITS rDNA sequence had no nucleotide differences in comparison with the S. hermaphroditum (JQ687355). However, one variation in the D2–D3 segment of 28S rDNA was observed in comparison with the AY598358. The phylogenetic analysis using ITS and 28S rDNA indicated that the Indian S. hermaphroditum could be placed together with other S. hermaphroditum, with strong posterior probability. Besides, the PCA analysis demonstrated some variability within the test populations. The distribution of S. hermaphroditum based on meta-analysis of the GenBank records showed its presence in the three Asian countries—India, Thailand and Indonesia. The Indian strain of S. hermaphroditum also tested positively for its virulence against three major pests, namely, Galleria mellonella, Helicoverpa armigera, and Spodoptera litura, with resultant which showed good efficacy on the mortalities.
Conclusions
In conclusion, the economy of India is agriculture-based, but there are huge losses due to different insect pests infesting different crops. Steinernema hermaphroditum CS34 is an indigenous species to Indian subcontinent and efforts should be made to evaluate its virulence and pathogenicity against the other agricultural pests hampering productivity throughout the country. This may lead to incorporate S. hermaphroditum strain CS34 as a regular biological control agent against important lepidopteran pest in integrated pest management programs in the future.




Similar content being viewed by others
References
Aasha, Chaubey AK, Bhat AH (2019) Notes on Steinernema abbasi (Rhabditida: Steinernematidae) strains and virulence tests against lepidopteran and coleopterans pests. J Entomol Zool Stud 7(2):954–964
Achinelly MF, Eliceche DP, Belaich MN, Ghiringhelli PD (2017) Variability study of entomopathogenic nematode populations (Heterorhabditidae) from Argentina. Braz J Biol 77(3):569–579
Adams BJ, Burnell AM, Powers TO (1998) A phylogenetic analysis of Heterorhabditis (Nemata: Rhabditidae) based on internal transcribed spacer 1 DNA sequence data. J Nematol 30:22–39
Addinsoft (2007) XLSTAT. Analyse de données et statistique avec MS Excel, Addinsoft
Ali SS, Azra S (2011) Steinernema sayeedae sp. n. a heat tolerant EPN from banana rhizosphere of Koshambhi district, U.P. India Trends Biosci 4:123–125
Ali SS, Riyaz A, Verma V, Azra S, Rashid P, Sobia A (2010) Molecular characterization of Steinernema masoodi, S. seemae and other Indian isolates of Steinernema spp. Trends Biosci 3:112–116
Ali SS, Shaheen A, Asif M, Akhtar MH (2009) Steinernema qazii sp. n. (Nematoda: Steinernematidae) from Kanpur, India. Trends Biosci 2:59–64
Ali SS, Shaheen A, Pervez R, Hussain MA (2005) Steinernema masoodi sp. n. and S. seemae sp. n. (Nematoda: Rhabditidae [sic]: Steinernematidae) from India. Int J Nematol 15:89–99
Banu JG, Nguyen KB, Rajendran G (2005) Occurrence and distribution of entomopathogenic nematodes in Kerala, India. Int J Nematol 15:9–16
Balasubramanian N, Toubarro D, Nascimento G, Ferreira R, Simoes N (2012) Purification, molecular characterization and gene expression analysis of an aspartic protease (Sc-ASP113) from the nematode Steinernema carpocapsae during the parasitic stage. Mol Biochem Parasitol 182:37–44
Balasubramanian N, Toubarro D, Simoes N (2010) Biochemical study and in vitro insect immune suppression by a trypsin-like secreted protease from the nematode Steinernema carpocapsae. Parasit Immunol 32:165–175
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21:109–110
Bhat AH, Bharti L, Istkhar, Aasha, Chaubey AK (2016) Phylogenic, pathogenic and reproductive characterization of Heterorhabditis indica from district Meerut, India. Int J Pharm Biol Sci 6:60–73
Bhat AH, Chaubey AK, Půža V (2018) The first report of Xenorhabdus indica from Steinernema pakistanense: co-phylogenetic study suggests co-speciation between X. indica and its steinernematid nematodes. J Helminthol 92:1–10
Bhat AH, Chaubey AK, Upadhyay SK (2016) Morphotaxometric and molecular validation of entomopathogenic nematode, Steinernema abbasi (rhabditida: steinernematidae) with mucronate processes in adults of second generations off subhumid region, Uttar Pradesh, India. World J Pharm Pharmaceut Sci 5:1558–1579
Bhat AH, Istkhar, Chaubey AK, Půža V, San-Blas E (2017) First report and comparative study of Steinernema surkhetense (Rhabditida: Steinernematidae) and its symbiont bacteria from subcontinental India. J Nematol 49:92–102
Bonner TP (1979) Initiation of development in vitro of 3rd-stage Nippostrongylus brasiliensis. J Parasitol 65:74–78
Campos-Herrera R, Escuer M, Robertson L, Gutierrez C (2006) Morphological and ecological characterization of Steinernema feltiae (Rhabditida: Steinernematidae) Rioja strain isolated from Bibio hortulanus (Diptera: Bibionidae) in Spain. J Nematol 38:68–75
Courtney WD, Polley D, Miller VL (1955) TAF, an improved fixative in nematode technique. Plant Dis Rep 39:570–571
Dolinski C, Kamitani F, Machado I, Winter C (2008) Molecular and morphological characterization of heterorhabditid entomopathogenic nematodes from the tropical rainforest in Brazil. Mem Inst Oswaldo Cruz 103:150–159
Elawad AS, Ahmad W, Reid AP (1997) Steinernema abbasi sp. n. (Nematoda: Steinernematidae) from the Sultanate of Oman. Fundam Appl Nematol 20:435–442
Filipjev IN (1934) Miscellanea Nematologica 1. Eine neue Art der Gattung Neoaplectana Steiner nebst Bermerkungen über die systematische Stellung der letzteren. Parazitologichesky 4:229–240
Forschler BT, Nordin GL (1988) Comparative pathogenecity of selected entomogenous to the hardwood borers, Prionoxystus robiniae (Lepidoptera: Cosidae) and Megacyllene robiniae (Coleoptera: Cerambicidae). J Invertebr Pathol 52:343–347
Ganguly S, Rathore KS, Sushil K, Singh M (2011) Steinernema meghalayensis sp. n. (Rhabditida: Steinernematidae) from northeastern hilly region of India. Indian J Nematol 41:83–97
Ganguly S, Singh LK (2000) Steinernema thermophilum sp. n. (Rhabditida: Steinernematidae) from India. Int J Nematol 10:183–191
Ganguly S, Singh M, Lal M, Singh LK, Vyas RV, Patel DJ (2002) New record of an entomopathogenic nematode, Steinernema riobrave Cabanillas, Poinar & Raulston, 1994 from Gujarat, India. Indian J Nematol 32:223
Golden JW, Riddle DLA (1982) A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218:578–580
Griffin CT, Chaerani R, Fallon D, Reid AP, Downes MJ (2000) Occurrence and distribution of the entomopathogenic nematodes Steinernema spp. and Heterorhabditis indica in Indonesia. J Helminthol 74:143–150
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucl Acids Symp Ser 41:95–98
Harris NC, Coonan TJ, King JL, Dunn RR (2013) Endemism in host–parasite interactions among island populations of an endangered species. Divers Distrib 19:377–387
Hawdon JM, Volk SW, Pritchard DI, Schad GA (1992) Resumption of feeding in vitro by hookworm 3rd-stage larvae—a comparative study. J Parasitol 78:1036–1040
Hominick WM (2002) Biogeography. In: Entomopathogenic nematology (Gaugler R ed.). Wallingford, UK: CABI Publishing, 115–143
Hunt D (2007) Overview of taxonomy and systematics. In: Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Nematology monographs and perspectives (Nguyen KB and Hunt DJ eds.). Leiden, the Netherlands, Brill Publishing. 5, 27–57
Hunt DJ, Subbotin SA (2016) Taxonomy and systematics. In: Advances in entomopathogenic nematode taxonomy and phylogeny (Nguyen HB and Hunt DJ eds.). Leiden, the Netherlands, Brill Publishing, 13–58
Hussaini SS, Ansari MA, Ahmad W, Subbotin SA (2001) Identification of some Indian populations of Steinernema species (Nematoda) by RFLP analysis of ITS region of rDNA. Int J Nematol 11:73–76
Kadav A, Lalramliana A (2012) Efficacy of indigenous entomopathogenic nematodes from Meghalaya, India against the larvae of taro leaf beetle, Aplosonyx chalybaeus (Hope). J Parasit Dis 36:149–154
Kalia V, Sharma G, Shapiro-Ilan DI, Ganguly S (2014) Biocontrol potential of Steinernema thermophilum and its symbiont Xenorhabdus indica against lepidopteran pests: virulence to egg and larval stages. J Nematol 46:18–26
Kaya HK, Gaugler R (1993) Entomopathogenic Nematodes. Annu Rev Entomol 38:181–206
Khatri-Chhetri HB, Waeyenberge L, Spiridonov S, Manandhar HK, Moens M (2011) Two new species of Steinernema Travassos, 1927 with short infective juveniles from Nepal. Russ J Nematol 19:53–74
Khatri-Chhetri HB, Waeyenberge L, Spiridonov SE, Manandhar HK, Moens M (2011) Steinernema lamjungense n. sp. (Rhabditida: Steinernematidae), a new species of entomopathogenic nematode from Lamjung district. Nepal. Nematology 13:589–605
Kulkarni N, Rizvi AN, Kumar V, Paunikar S, Mishra VK (2012) Morphological and molecular characterization of Steinernema dharanaii sp. n.: a new entomopathogenic nematode from India. Indian J Trop Biodiver 20:107–116
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lalramnghaki HC, Vanlalhlimpuia Vanramliana, Lalramliana A (2017) Characterization of a new isolate of entomopathogenic nematode, Steinernema sangi (Rhabditida, Steinernematidae), and its symbiotic bacteria Xenorhabdus vietnamensis (c-Proteobacteria) from Mizoram, north-eastern India. J Parasit Dis 41:1123–1131
Mhatre PH, Patil J, Kumar RV, Venkatasalam EP, Divya KI, Jenifer J, Pankaj Chavan S (2017) The first report of Steinernema cholashanense (Rhabditida: Steinernematidae) from India. Indian J Nematol 47:254
Lewis EE, Gaugler R, Harrison R (1992) Entomopathogenic nematode host-finding: response to host contact cues by cruise and ambush foragers. Parasitology 105:309–315
Lu D, Macchietto M, Chang D, Barros MM, Baldwin J, Mortazavi A, Dillman AR (2017) Activated entomopathogenic nematode Infective juveniles release lethal venom proteins. PLoS Pathog 13:e1006302. https://doi.org/10.1371/journa.ppat.1006302
Lu D, Sepulveda C, Dillman AR (2016) Infective juveniles of the entomopathogenic nematode Steinernema scapterisci are preferentially activated by cricket tissue. PLoS One 12:e0169410. https://doi.org/10.1371/journal.pone.0169410
Luc PV, Nguyen KB, Reid AP, Spiridonov SE (2000) Steinernema tami sp. n. (Rhabditida: Steinernematidae) from Cat Tien Forest, Vietnam. Russ J Nematol 8:33–43
Nadler SA, Bolotin E, Stock SP (2006) Phylogenetic relationships of Steinernema Travassos, 1927 (Nematoda: Cephalobina: Steinernematidae) based on nuclear, mitochondrial and morphological data. Syst Parasitol 63:161–181
Nguyen KB, Půža V, Mráček Z (2008) Steinernema cholashanense n. sp. (Rhabditida, Steinernematidae) a new species of entomopathogenic nematode from the province of Sichuan, Chola Shan Mountains, China. J Invert Patholol 97:251–264
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Pervez R, Ali SS, Asif M (2009) A new species of entomopathogenic nematodes Steinernema mushtaqi sp. n. (Nematoda: Rhabditida: Steinernematidae) from chickpea rhizosphere. In: International Conference on Legumes (ICGL), Indian Institute of Pulses Research, Kanpur, February, 14–16
Phan KL, Nguyen NC, Moens M (2001) Steinrnema sangi sp. n. (Rhabditida: Steinernematidae) from Vietnam. Russ J Nematol 9:1–7
Phan KL, Subbotin SA, Nguyen NC, Moens M (2003) Heterorhabditis baujardi sp. n. (Rhabditida: Heterorhabditidae) from Vietnam and morphometric data for H. indica populations. Nematology 5:367–382
Poinar GO Jr (1976) Description and biology of a new insect parasitic rhabditoid, Heterorhabditis bacteriophora n. gen. n. sp. (Rhabditida; Heterorhabditidae n. fam.). Nematologica 21:463–470
Poinar GO Jr, Karunakar GK, David H (1992) Heterorhabditis indicus n. sp. (Rhabditida, Nematoda) from India: separation of Heterorhabditis spp. by infective juveniles. Fundam Appl Nematol 15:467–472
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Qiu L, Fang Y, Zhou Y, Pang Y, Nguyen KB (2004) Steinernema guangdongense sp. n. (Nematoda: Steinernematidae), a new entomopathogenic nematode from southern China with a note on S. serratumn (nomen nudum). Zootaxa 704:1–20
Qiu L, Zhao J, Wu Z, Lv Z, Pang Y (2011) Steinernema pui sp. n. (Rhabditida, Steinernematidae), a new entomopathogenic nematode from Yunnan, China. Zootaxa 2767:1–13
Ronquist F., Huelsenbeck J., Teslenko M. 2011. Draft MrBayes version 3.2 Manual: Tutorials and Model Summaries. Distributed with the software from mrbayes.sourceforge.net/mb3.2_manual.pdf
Seenivasan N, Prabhu S, Makesh S, Sivakumar M (2012) Natural occurrence of entomopathogenic nematode species (Rhabditida: Steinernematidae and Heterorhabditidae) in cotton fields of Tamil Nadu, India. J Nat Hist 46:2829–2843
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerine. Nematologica 4:67–69
Shapiro-Ilan DI, Stuart DI, McCoy CW (2003) Comparison of beneficial traits among strains of the entomopathogenic nematode, Steinernema carpocapsae, for control of Curculio caryae (Coleoptera: Curculionidae). Biol Control 28:129–136
Shen CP, Wang GH (1992) Description of an entomopathogenic nematode, Steinernema longicaudum sp. nov. and its application. In: Proceedings of the XIX international congress of entomology, Beijing, China, pp. 220–231
Sivakumar CY, Jayaraj S, Subramanian S (1989) Observations on an Indian population of the entomopathogenic nematode, Heterorhabditis bacteriophora Poinar, 1976. J Biol Control 2:112–113
Steiner G (1929) Neoaplectana glaseri n.g., n.sp. (Oxyuridae) a new nemic parasite of the Japanese beetle. J Wash Acad Sci 19:436–440
Stock SP, Griffin CT, Chaerani R (2004) Morphological and molecular characterisation of Steinernema hermaphroditum n. sp. (Nematoda: Steinernematidae), an entomopathogenic nematode from Indonesia, and its phylogenetic relationships with other members of the genus. Nematology 6:401–412
Stock SP, Somsook V, Reid A (1998) Steinernema siamkayai n. sp. (Rhabditida: Steinernematidae), an entomopathogenic nematode from Thailand. Syst Parasitol 41:105–113
Tallosi B, Ehlers R (1995) Steinernema bicornutum sp. n. (Rhabditida: Steinernematidae) from Vojvodina, Yugoslavia. Russ J Nematol 3:71–80
Vanlalhlimpuia, Lalramliana, Lalramnghaki HC, Vanramliana A (2018) Morphological and molecular characterization of entomopathogenic nematode, Heterorhabditis baujardi (Rhabditida, Heterorhabditidae) from Mizoram, northeastern India. J Parasit Dis 7:6. https://doi.org/10.1007/s12639-018-1004-0
Vrain TC, Wakarchuk DA, Levesque AC, Hamilton RI (1992) Intraspecific rDNA restriction fragment length polymorphisms in the Xiphinema americanum group. Fundam Appl Nematol 15:563–574
Weiser J (1955) Neoaplectana carpocapsae n. sp. (Anguillata, Steinernematidae) novy cizopasnik housenek obalece jablecneho, Carpocapsae pomonella L. Vestnik Ceskoslovenske Spolecnosti Zoologicke 19:44–52
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Wouts WM, Mráček Z, Gerdin S, Bedding RA (1982) Neoaplectana Steiner, 1929 a junior synonym of Steinernema Travassos, 1927 (Nematoda: Rhabditida). Syst Parasitol 4:147–154
Acknowledgements
We thank the Head of the Department of Zoology, Chaudhary Charan Singh University, Meerut, India, for providing necessary facilities for conducting the experiments.
Funding
The authors thank the Department of Science and Technology (DST), New Delhi, India, for providing financial assistance through DST INSPIRE Fellowship/2014/76.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhat, A.H., Chaubey, A.K., Shokoohi, E. et al. Study of Steinernema hermaphroditum (Nematoda, Rhabditida), from the West Uttar Pradesh, India. Acta Parasit. 64, 720–737 (2019). https://doi.org/10.2478/s11686-019-00061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00061-9