A Short Commentary on Potential Role of the IFN-γ/mini-TrpRS Signaling Axis in Abdominal Aortic Aneurysm
Article Information
Corey S Moran1, Ramesh B Velu2, Venkat Vangaveti1,3, Usman H Malabu1,3,4, Erik Biros1*
1College of Medicine and Dentistry, James Cook University, Townsville, Queensland, Australia
2Department of Vascular and Endovascular Surgery, Townsville University Hospital, Townsville, Queensland, Australia
3Australian Institute of Tropical Health and Medicine, James Cook University, Townsville, Queensland, Australia
4Townsville University Hospital, Townsville, Queensland, Australia
*Corresponding author: Erik Biros, College of Medicine and Dentistry, James Cook University, Townsville, Queensland, Australia, 4811
Received: 05 July 2020; Accepted: 17 July 2020; Published: 10 August 2020
Citation: Corey S Moran, Ramesh B Velu, Venkat Vangaveti, Usman H Malabu, Erik Biros. A Short Commentary on Potential Role of the IFN-γ/mini-TrpRS Signaling Axis in Abdominal Aortic Aneurysm. Archives of Clinical and Biomedical Research 4 (2020): 361-366.
View / Download Pdf Share at FacebookAbstract
Aneurysms demonstrate vascular smooth muscle cells (VSMC) phenotypic modulation characterized by the prevailing of synthetic over contractile properties. We have shown previously the ability of interferon-gamma (IFN-γ) to switch VSMC from contractile to synthetic phenotype via upregulating the truncated form of tryptophanyl-tRNA synthetase (mini-TrpRS) in vitro. Here we discuss a possible role of the IFN-γ/mini-TrpRS signalling axis in the pathology of human abdominal aortic aneurysm (AAA).
Keywords
Mini tryptophanyl-tRNA synthetase; Interferon-gamma; Abdominal aortic aneurysm
Mini tryptophanyl-tRNA synthetase articles, Interferon-gamma articles, Abdominal aortic aneurysm articles
Article Details
The Background
Abdominal aortic aneurysm (AAA) is a common cause of chronic morbidity in the elderly population associated with more than 90% mortality when it ruptures [1]. Aneurysm rupture is mainly attributed to the weakening of the aortic wall associated with the loss of stabilizing structures and smooth muscle cell contractility [2]. Vascular smooth muscle cells (VSMC) are the predominant cell-type within the thickest layer of the aortic wall that regulates pulse pressure and force distribution due to their contractile properties and linkage with the extracellular matrix [3]. Dysregulated positive remodelling of the artery wall characterizing AAA involves the dedifferentiation of VSMC [4]. Although phenotypic modulation of VSMC between their quiescent contractile and active synthetic phenotype is a fundamental property of these cells in regulating the aortic wall integrity, mechanisms leading to dysregulation of this basic cell phenomenon in AAA pathophysiology is poorly understood.
Several traditional risk factors have been linked to AAA, including smoking, ageing, hypertension, hypercholesterolemia, and family history [5]; however, there is clear evidence that inflammatory pathways are principal and common pathogenic mediators in the course of AAA [6] under the stimuli of traditional risk factors. The proinflammatory cytokine interferon (IFN)-γ is a classic representative of inflammatory mediators that are expressed at high levels in AAA [7]. Biological effects of IFN-γ on VSMC contributing to vascular remodelling have been described [8-10], requiring a shift from a quiescent contractile phenotype to an activated synthetic phenotype. In a recent study, we identified a novel indirect molecular mechanism by which IFN-γ elicits VSMC phenotypic modulation that opens a promising non-immunomodulatory treatment option for patients with AAA [11].
The Problem: Impaired VSMC contractility is a novel concept to explain the occurrence and progression of AAA [2]. Innovations in controlling the phenotypic modulation of VSMC would lead to asignificant improvement in the clinical management of AAA.
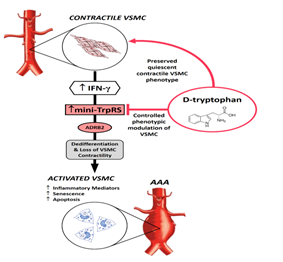
The Rationale and Solution: Interferon-gamma (IFN-γ), a pleiotropic cytokine critical for innate and adaptive immunity, stimulates an inflammatory response in VSMCs requiring a shift from a quiescent contractile phenotype to an activated synthetic phenotype [10]. IFN-γ controls cellular processes through transcriptional regulation of its dependent genes. For instance, IFN-γ immediately causes transcriptional stimulation of mini-tryptophanyl-tRNA synthetase (mini-TrpRS) that represents the truncated form of the cytoplasmic full-length TrpRS enzyme [12,13]. Interestingly, mini-TrpRS is the only aminoacyl-tRNA synthetase that is transcriptionally inducible by IFN-γ [14-20]. The biological significance of this phenomenon lies in the fact that mini-TrpRS exerts potent signalling actions outside its canonical role in protein synthesis in which mini-TrpRS enzymatically attaches its cognate amino acid tryptophan (Trp) onto the transfer ribonucleic acid tRNATrp. In our experiments, a characteristic epithelioid (synthetic) phenotype of VSMCs activated with IFN-γ [8] corresponded with the remarkable up-regulation of mini-TrpRS [21]. Importantly, we discovered that blockade of mini-TrpRS with D-tryptophan, acting as a decoy substrate to prevent mini-TrpRS signalling, caused a phenotypic switch to a spindle-shaped (contractile) VSMC morphology in the presence of IFN-γ in vitro, identifying mini-TrpRS to be essential for a mechanism by which IFN-γ indirectly controls phenotypic modulation of VSMC [11]. An important question, whether the IFN-γ/mini-TrpRS signalling axis would be involved in remodelling and weakening of aortic wall at a point in AAA progression in patients, is posed. This hypothesis is holding for data where elevated levels of IFN-γ predict an increased rate of AAA expansion [7], wherein AAA diameter is a strong predictor of rupture [22]. Mini-TrpRS signalling inevitably occurs in the context of more complex regulation mediated by IFN-γ with the ability to execute specific outcomes depending on the pathological environment and the cell type involved. Thus, a direct blockade of mini-TrpRS with its cognate amino acid D-tryptophan would preserve a quiescent contractile phenotype of VSMC, therefore limit impaired contractility of VSMC in the course of AAA and hold promising non-immunomodulatory treatment option for patients with AAA. In this context, in animal models, D-tryptophan is readily cleared from plasma, and there is no appreciable conversion of D-tryptophan to metabolically active L-tryptophan [23]. D-tryptophan is an enantiomer of L-tryptophan, where the only difference between these two forms of tryptophan is the orientation of the molecule. The essential amino acid L-tryptophan is found in most human proteins and must be resourced from the diet. L-tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin and vitamin B3 [24]. The therapeutic use of L-tryptophan has previously focused on mood-affective disorders with doses up to 6 g/day being considered safe and generally free of side effects [25]. D-tryptophan, unlike L-tryptophan, is not a constituent of human proteins and has no metabolic or biological functions attributable to L-tryptophan. Consequently, side effects such as unintended stimulation of serotonin production in the brain [23] or other metabolic functions of the L-tryptophan [25] would be expected to be negligible in treated AAA patients. The proposed model for the treatment of AAA using D-tryptophan is shown in Figure 1. Studies investigating IFN-γ/mini-TrpRS signalling and suggested intervention with D-tryptophan in AAA is planned for future work.
Figure 1: Proposed AAA therapy based on D-tryptophan. Interferon-γ (IFN-γ) is chronically elevated in AAA. This inevitably up-regulates mini-TrpRS that promotes de-differentiation and loss of VSMC contractility. Intervention with D-tryptophan would prevent the dedifferentiation of VSMC controlling phenotypic modulation of these cells in the course of AAA, thus regulating AAA expansion. ADRB2, β2-adrenoceptor.
Acknowledgments
This work was supported by the College of Medicine and Dentistry, James Cook University.
Author Contributions
Conceptualization, E.B. and C.S.M.; Writing – Original Draft Preparation, E.B. and C.S.M.; Writing – Review & Editing, R.B.V., U.H.M., and V.V.; Visualization, C.S.M.; Funding Acquisition, R.B.V. and U.H.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgraduate Medical Journal 85 (2009): 268-273.
- Bogunovic N, Meekel JP, Micha D, et al. Impaired smooth muscle cell contractility as a novel concept of abdominal aortic aneurysm pathophysiology. Scientific Reports 9 (2019): 6837.
- Milewicz DM, Guo DC, Tran-Fadulu V, et al. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genomics Hum. Genet. 9 (2008): 283-302.
- Forsdahl SH, Singh K, Solberg S, et al. Risk factors for abdominal aortic aneurysms. Circulation 119 (2009): 2202-2208.
- Li H, Bai S, Ao Q, et al. Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets. Journal of Immunology Research 2018 (2018): 7213760.
- Juvonen J, Surcel HM, Satta J, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology 17 (1997): 2843-2847.
- Biros E, Moran CS. Mini tryptophanyl-tRNA synthetase is required for a synthetic phenotype in vascular smooth muscle cells induced by IFN-γ-mediated β2-adrenoceptor signaling. Cytokine 127 (2020): 154940.
- Tolstrup AB, Bejder A, Fleckner J, et al. Transcriptional regulation of the interferon--inducible tryptophanyl-tRNA synthetase includes alternative splicing. Journal of Biological Chemistry 270 (1995): 397-403.
- Turpaev KT, Zakhariev VM, Sokolova IV, et al. Alternative processing of the tryptophanyl-tRNA synthetase mRNA from interferon-treated human cells. European Journal of Biochemistry 240 (1996): 732-737.
- Fleckner J, Rasmussen HH, Justesen J. Human interferon gamma potently induces the synthesis of a 55-kDa protein (gamma 2) highly homologous to rabbit peptide chain release factor and bovine tryptophanyl-tRNA synthetase. Proceedings of the National Academy of Sciences 88 (1991): 11520-11524.
- Rubin BY, Anderson SL, Xing L, et al. Interferon induces tryptophanyl-tRNA synthetase expression in human fibroblasts. Journal of Biological Chemistry 266 (1991): 24245-24248.
- Kisselev L, Frolova L, Haenni AL. Interferon inducibility of mammalian tryptophanyl-tRNA synthetase: new perspectives. Trends in Biochemical Sciences 18 (1993): 263-267.
- Reano A, Richard MH, Denoroy L, et al. Gamma interferon potently induces tryptophanyl-tRNA synthetase expression in human keratinocytes. Journal of Investigative Dermatology 100 (1993): 775-779.
- Fleckner J, Martensen PM, Tolstrup AB, et al. Differential regulation of the human, interferon inducible tryptophanyl-tRNA synthetase by various cytokines in cell lines. Cytokine 7 (1995): 70-77.
- Shaw AC, Røssel Larsen M, Roepstorff P, et al. Mapping and identification of interferon gamma-regulated HeLa cell proteins separated by immobilized pH gradient two-dimensional gel electrophoresis. Electrophoresis: An International Journal 20 (1999): 984-993.
- Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284 (1999): 147-151.
- Robinson D, Mees B, Verhagen H, et al. Aortic aneurysms: Screening, surveillance and referral. Australian Family Physician 42 (2013): 364.
- Budny J, Dow RC, Eccleston D, et al. A comparison of D and L-tryptophan on the cerebral metabolism of 5-hydroxytryptamine and dopamine in the dog. British Journal of Pharmacology 58 (1976): 3.
- Slominski A, Semak I, Pisarchik A, et al. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Letters 511 (2002): 102-106.
- Richard DM, Dawes MA, Mathias CW, et al. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int J Tryptophan Res 2 (2009): 45-60.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 