Demographic and Clinical Characteristics of Hypertensive and/or Diabetic Patients with Target Organ Damage in the Lebanese Population
Article Information
Jeannot Kekedjian1†, Raymond Challita1†, Mikael Abi Abdallah1†, Roland Asmar1,2, Mirna N Chahine1,2*
† Authors’ equal contribution
1 Faculty of Medical Sciences, Lebanese University, Hadath, Lebanon
2 Foundation-Medical Research Institutes (F-MRI®), Beirut, Lebanon
*Corresponding author: Prof. Mirna N Chahine, Lebanese University, Faculty of Medical Sciences Hadath, Lebanon
Received: 22 October 2020; Accepted: 30 October 2020; Published: 10 November 2020
Citation: Jeannot Kekedjian, Raymond Challita, Mikael Abi Abdallah, Roland Asmar, Mirna N Chahine. Demographic and Clinical Characteristics of Hypertensive and/or Diabetic Patients with Target Organ Damage in the Lebanese Population. Archives of Clinical and Biomedical Research 4 (2020): 361-377.
View / Download Pdf Share at FacebookAbstract
Background: Hypertension and diabetes represent a major public health problem. They are associated with complications, organ damage, and an increase in cardiovascular mortality. Objective: The aim of this study was to analyze the demographic and clinical characteristics of hypertensive and/or diabetic patients with target organ damage in the Lebanese population.
Methods: Retrospective cross-sectional multicentric study conducted on 750 Lebanese patients randomly selected from 6 hospitals from January 2014 to August 2015. Patients were divided according to their medical history: 252 hypertensive, 249 diabetic, 249 both hypertensive and diabetic. All patients had target organ damages and were admitted to the hospitals.
Results: Our study showed the following demographic characteristics: men were affected more than women; young men and older women being more commonly affected in each gender subgroup; hypertension was associated with older age compared to diabetes. It also showed the following clinical data: hypertension was associated with higher risk of cardiovascular diseases, higher systolic blood pressure, and left ventricular hypertrophy; diabetes was associated with higher incidence of nephropathy, dyslipidemia and cardiovascular diseases (if positive family history); having both diseases was associated with developing atherosclerotic plaques.
Conclusion: Our study demonstrated an increased prevalence of affected Lebanese men (by both diseases) in the younger population, and increased prevalence of Lebanese women in the older population (> 75 years old). Family history of previous cardiovascular diseases was present in all groups. Disease recurrence, as defined in our study by the number of existing cardiovascular diseases, was more common in patients with both diseases.
Keywords
Hypertension; Diabetes; Lebanon; Target organ damage; Demographic
Article Details
1. Introduction
Hypertension (HTN) and diabetes mellitus (DM) represent a major public health problem nowadays. They are associated with multiple complications, organ damage, and an increase in cardiovascular mortality [1]. According to the American Heart Association (AHA), HTN affects 103 million US adults and its prevalence will continue to increase to reach 41.4% of the US adults in 2030. Moreover, 23.4 million adults have diagnosed DM, 81.6 million adults have pre DM, and 7.6 million adults have undiagnosed DM in the US. This prevalence varies by sex and ethnicity [2].
In Lebanon, the most commonly identified health problems are HTN and DM [3]. A strong correlation exists between HTN, DM and cardiovascular diseases (CVD): HTN and its treatment are associated with late coronary artery diseases (CAD) [4], while DM patients are thirty-two times more prone to have cardiac events than non-diabetics [5]. Lebanon has witnessed a threefold increase in the prevalence of HTN during the past decade [6], and currently, the prevalence of DM has also increased to 15% [7]. The burdens of the disease can be due to the changes in the demographic characteristics and the population lifestyle [6]. This is dangerous because the presence of any of the four markers of organ damage, that are microalbuminuria, increased pulse wave velocity, left ventricular hypertrophy (LVH), and carotid plaques, can predict cardiovascular mortality independently, and this risk increases with the number of damaged organs [8].
In the States, only one fourth of the hypertensive patients have their HTN controlled [9], and two third of them have their DM controlled [10]. Indeed, various demographic characteristics were studied as a potential factor affecting both HTN [9] and DM [11] treatment control. Many studies described the prevalence of HTN and DM worldwide and in Lebanon [12, 13], while others tried to study the demographic characteristics in these patients. However, none was reported about patients with DM and /or HTN with associated target organ damage in Lebanon. We therefore conducted this study to analyze the demographic characteristics, to identify the profiles, and to reveal the differences, if any, in this specific population.
2. Subjects and Methods
2.1 Study design and study population
The study followed a stratified study design. It was a cross-sectional multicentric study that included 750 Lebanese patients randomly selected from 6 hospitals: the academic Beirut Governmental University Hospital, Sacré-Coeur Hospital, Lebanese Geitaoui Hospital, Middle East Institute of Health-Bsalim Hospital, Mount-Lebanon Hospital, and Notre Dame du Liban Hospital, from January 2014 to August 2015. An IRB approval was obtained from these hospitals before beginning the study. Data was then collected and patients were grouped based on the information retrieved from their previous medical history section in their medical file.
Group 1: consisted of 252 patients known to have HTN.
Group 2: consisted of 249 patients known to have DM.
Group 3: consisted of 249 patients known to have HTN and DM.
Patients with HTN and having one or more of the TOD and/or previous CVD; Patients with DM and having one or more of TOD and/or previous CVD, and Patients with DM and HTN and having one or more of the TOD and/or previous CVD were included in this study.
However, patients with no HTN and/or no DM, no TOD, and no CVD were excluded from the study.
2.2 Data collection
Collection of data started in January 2014 and ended in August 2015. For each patient, a case report form was filled out including parameters of gender, date of birth, and the visit date as well as the following criteria:
- History of CVD: Cerebrovascular, cardiac, renal, vascular….
- CV risk factors: men older than 55 or female older than 65, smoker, family history of CVD (before 65), obesity, HTN, dyslipidemia, and DM.
- TOD: LVH, proteinuria, atherosclerotic plaques.
- Clinical examination: height, weight, Body mass Index (BMI), blood pressure (BP), Waist circumference, etc.
2.3 Statistical analysis
Data analysis was completed using SPSS version 22. Descriptive statistics were used to calculate the frequency, mean and standard deviation (SD) of the continuous variables and the frequency and percentages of the nominal variables. Bivariate analysis was used to assess the correlation between the study groups and all the study variables using Chi-Square Test, Fisher’s Exact Test, Student t-test, and ANOVA test. Two-sided p-values were calculated in all tests, with statistical significance set at p < 0.05.
3. Results
3.1 Demographic characteristics
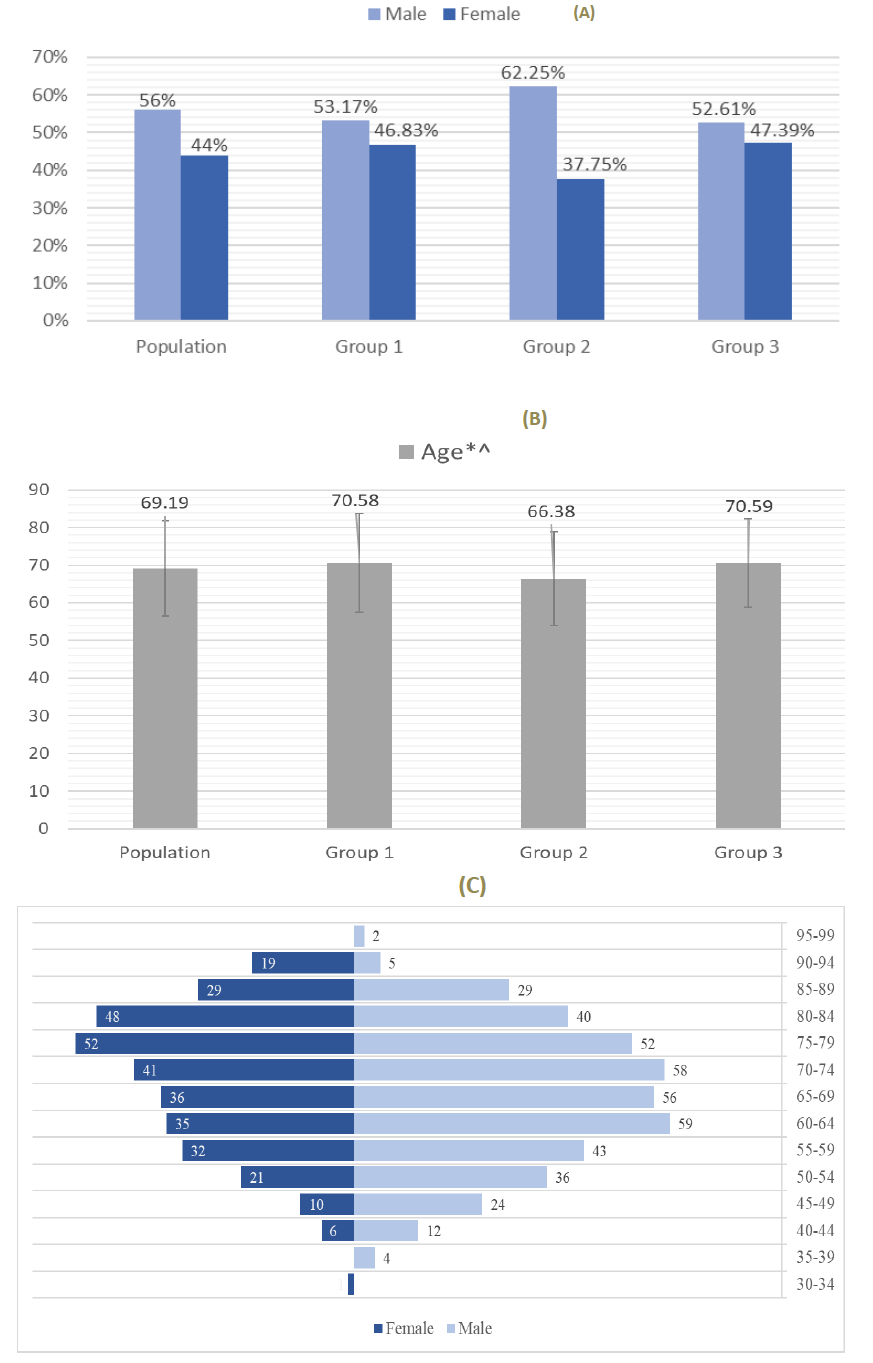
3.1.1 Gender distribution: The study included 750 patients, 420 (56%) men and 330 (44%) women, distributed between the 3 groups as shown in Figure 1A. The group 1 included 252 people with HTN, 134 (53.2%) men and 118 (46.8%) women. The group 2 included 249 people with DM, 155 (62.2%) men and 94 (37.8%) women. The group 3 included 249 people with both HTN and DM, 131 (52.6%) men and 118 (47.4%) women (Figure 1A).
3.1.2 Age distribution: The median age was 69.19-year-old for the studied population. Patients of group 2 (66.38-year-old) had a significantly lower median age than patients of group 1 (70.58-year-old; p< 0.001) and group 3 (70.59-year-old; p< 0.001). There was no statistical difference between age of patients from group 1 and group 3 (Figure 1B). The most common age group for men and women was 60-64 years and 75-79 years, respectively (Figure 1C).
3.2 History of cardiovascular diseases
Cardiac diseases (76.9%) were the most common CVD present in the population followed by vascular diseases (71.9%), renal (41.5%), ‘’other’’ diseases, such as thyroid or respiratory diseases, 34.3%, and cerebrovascular diseases (13.7%). Frequency of cardiac diseases, cerebrovascular diseases, and ‘’others’’ did not significantly differ between patients of group 1, 2 or 3, meaning that patients with HTN, DM, or both with HTN and DM were equally associated to develop each of these diseases. However, the frequency of nephropathy was significantly higher in patients of group 2 and with patients of group 3 (37.6% vs 43.7 %; p< 0.001), compared with patients of group 1 (18.6%). These results showed that DM was associated with the development of nephropathy, compared with HTN alone. And both HTN and DM were associated with the occurrence of a nephropathy compared with HTN alone, but not compared with DM alone. This showed that DM might indeed represent a significant factor associated with the development of nephropathy.
In addition, the frequency of vascular pathologies was significantly higher in patients of group 2 (33%) and group 3 (38.4%) compared with patients of group 1 (28.5%), with significant difference among 3 groups (p= 0.01). These results showed that HTN, DM or both DM and HTN were associated with the development of vascular diseases (Figure 2A). Overall, in term of the number of the diseases cited above, the studied population had an average of 2.38±1.09 diseases per person; Patients of group 2 (2.45±0.98 diseases), and group 3 (2.68±1.09 diseases) had a significantly higher number of diseases than those of group 1 (2.02±1.09 diseases). Number of diseases in group 3 was significantly higher than the one of group 2 (p= 0.016). This showed that compared with HTN alone, DM is highly associated with a higher frequency of the diseases cited above, and together HTN and DM were even more associated with a higher risk to develop these diseases compared with HTN alone.
The study included 577 patients with cardiac diseases (76.9%), among which 95.3% having a history of angina, 87.0% having coronary artery disease, 39.34% having heart failure, and 22.8% having a prior myocardial infarction. There were no statistical differences in terms of the number of each of these diseases between the 3 groups. This means that patients with HTN, or DM, or HTN with DM were equally at risk for having myocardial infarction, or angina, coronary artery disease, or heart failure. The distribution of patients from Groups 1, 2, and 3 are shown in Figure 2B.
Population (N=750); Group 1: Hypertension (N=252); Group 2: Diabetes (N=249); Group 3: Hypertension and Diabetes (N=249). *: p < 0.05 group 1 vs group 2; “: p< 0.05 group 1 vs group 3; ^: p< 0.05 group 2 vs group 3. Cerebrovascular: stroke, transient ischemic accident, carotid stenosis; Cardiac: myocardial infarction, angina, coronary artery disease, heart failure; Renal: diabetic nephropathy, renal failure; Vascular: lower limb atherosclerosis, retinopathy; Other: sleep apnea syndrome, peripheral artery disease, peripheral vascular disease, diabetic neuropathy, hypothyroidism.
3.3 Cardiovascular risk factors
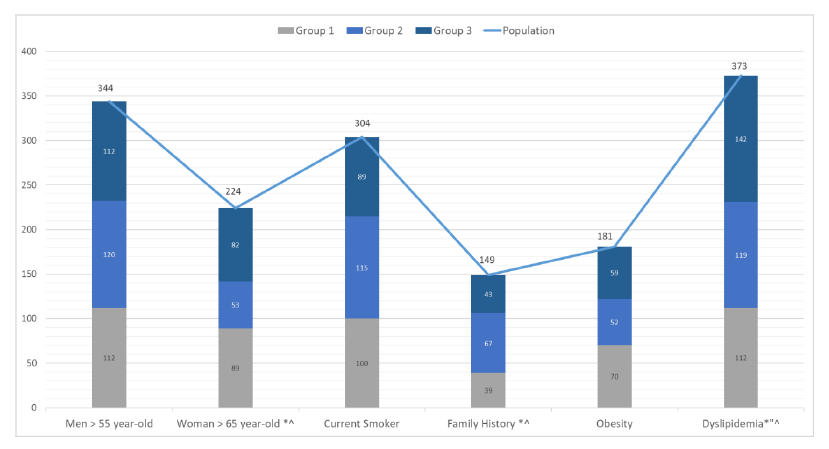
The most common CV risk factors in the population were males older than 55-year-old (81.9%), females older than 65-year-old (67.9%), dyslipidemia (49.7%), currently smoking (40.5%), obesity (24.1%), and a positive family history (19.9%). In our study, male patients above the age of 55, current smokers, and obese patients were evenly distributed with no statistical difference between the 3 groups. This means that patients with HTN alone, or DM alone, or both HTN and DM were equally at risk for developing CVD if they were males older than 55 years, or currently smoking or obese (Figure 3).
In females above the age of 65, the percentage of patients of group 2 (23.6%) was statistically lower than the percentage of patients of group 1 (39.7%, p< 0.001), and group 3 (36.6%, p= 0.003), which shows that females older than 65 years with HTN or with HTN and DM were at higher risk of CVD than those with DM. However, females older than 65 with HTN or HTN with DM were at equally at risk for developing CVD (Figure 3).
Population (N=750); Group 1: Hypertension (N=252); Group 2: Diabetes (N=249); Group 3: Hypertension and Diabetes (N=249). *: p < 0.05 group 1 vs group 2; “: p< 0.05 group 1 vs group 3; ^: p< 0.05 group 2 vs group 3.
In patients with a positive family history for cardiac diseases, the percentage of patients of group 2 (44.9%) was statistically higher than the percentage of patients of group 1 (26.1%, p=0.002), and group 3 (28.8%, p=0.010). These results show that compared with HTN alone, DM might be associated with a higher risk for developing CVD if they had a positive family history, but having both HTN and DM did not increase the risk for developing CVD if they had a positive family history. However, patients with HTN or HTN with DM were at equally at risk for developing CVD if they had a positive family history (Figure 3).
Finally, in patients with dyslipidemia, the percentages of patients of group 2 (31.9%), and group 3 (38.0%) were statistically higher than the percentage of patients of group 1 (30.0%) The percentage of patients of group 3 with dyslipidemia was also significantly higher than the one of group 2 (p= 0.010). This shows that compared with HTN alone, having DM or having both HTN and DM were associated with a higher risk of dyslipidemia.
3.4 Target organ damages
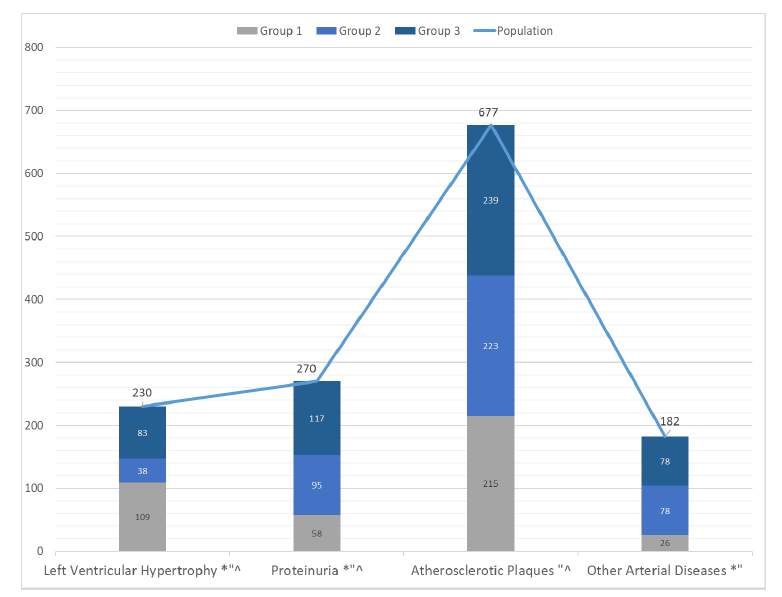
The most common target organ damages in the population were atherosclerotic plaques (90.3%), followed by proteinuria (36%), left ventricular hypertrophy (30.7%), and other arterial diseases (24.3%) (Figure 4).
Specifically, the frequency of atherosclerotic plaques was significantly higher in patients of group 3 (35.3 %) compared with patients of group 1 (31.7%; p< 0.001) and group 2 (32.9%; p= 0.006). This result shows that patients with both HTN and DM had a higher risk to develop atherosclerotic plaques, than those with HTN alone or DM alone. Also, patients with HTN alone and DM alone were at equal risk to develop atherosclerotic plaques (Figure 4).
As for the frequency of proteinuria, it was significantly higher in patients of group 3 (43.3 %; p< 0.001) and group 2 (35.1%; p< 0.001) compared with patients of group 1 (21.4%). There was also a higher frequency of proteinuria in patients with group 2 compared with group 1. This means that patients with DM and with both HTN and DM had a higher risk to have proteinuria than those with HTN. Also, patients with both HTN and DM had a higher risk to have proteinuria than those with DM alone (Figure 4).
Conversely, the frequency of left ventricular hypertrophy (LVH) was significantly higher in patients of group 1 (47.3 %) compared with patients of group 2 (16.5%; p< 0.001) and group 3 (36.0%; p= 0.022), and in patients with group 3 compared with the ones of group 2 (p< 0.001). This means that patients with HTN had a higher risk to have LVH than those with DM alone or HTN and DM. Also, patients with both DM and HTN were at a higher risk to have LVH than those with DM alone (Figure 4).
Population (N=750); Group 1: Hypertension (N=252); Group 2: Diabetes (N=249); Group 3: Hypertension and Diabetes (N=249). *: p < 0.05 group 1 vs group 2; “: p< 0.05 group 1 vs group 3; ^: p< 0.05 group 2 vs group 3.
Finally, the frequency of other arterial diseases was significantly higher in patients of group 2 (42.8%; p< 0.001) and group 3 (42.8%; p< 0.001), compared with patients of group 1 (14.2%). No statistical difference was shown in the frequency of other arterial diseases between patients with DM and with both DM and HTN. These results show that patients with DM or both DM and HTN had a higher risk to develop other arterial diseases than those with HTN alone. Also, patients with DM alone, or with both HTN and DM were equally at risk for developing other arterial diseases (Figure 4).
3.5 Clinical examination
The average Body Mass Index (BMI) was 27.7 kg/m2 and heart rate was 76.35 beats/min. There was no statistical difference between the 3 groups regarding their BMI and their heart rates (Table 1). The average Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) were 130.39mmHg and 72.8mmHg, respectively. SBP and DBP in patients of group 1 and group 3 were significantly higher compared with the ones in patients of group 2 (p< 0.001). However, there was no significant difference in SBP and DBP between patients of group 1 and 3. These results show that HTN alone, is associated with an increased BP, compared with DM (Table 1).
|
Population |
Group 1 |
Group 2 |
Group 3 |
|
|
Height (cm) *"^ |
168.12; 8.93 |
167.18; 8.96 |
169.66; 8.74 |
167.53; 8.93 |
|
Weight (kg) |
78.38; 17.93 |
78.29; 17.27 |
79.58; 19.24 |
77.27; 17.22 |
|
BMI (kg/m2) |
27.70; 6.06 |
27.95; 5.59 |
27.56; 6.08 |
27.60; 6.50 |
|
Systolic BP (mmHg) *^ |
130.39; 17.45 |
132.03; 17.67 |
124.81; 15.01 |
134.31; 18.14 |
|
Diastolic BP (mmHg) *^ |
72.80; 11.03 |
73.60; 10.98 |
70.35; 10.20 |
74.45; 11.49 |
|
Heart Rate (b/min) |
76.35; 10.15 |
76.01; 10.57 |
76.32; 9.94 |
76.73; 9.95 |
|
Total number of disease recurrence *"^ |
2.38; 1.09 |
2.02; 1.09 |
2.45; 0.98 |
2.68; 1.09 |
Table 1: Clinical examination.
Population (N=750); Group 1: Hypertension (N=252); Group 2: Diabetes (N=249); Group 3: Hypertension and Diabetes (N=249). *: p < 0.05 group 1 vs group 2; “: p< 0.05 group 1 vs group 3; ^: p< 0.05 group 2 vs group 3.
4. Discussion
In our study, 750 patients were selected according to their medical history, such as 252 hypertensive, 249 diabetic, 249 hypertensive and diabetic. All patients had TOD and were admitted patients. The studied parameters included age, sex, history of cardiovascular diseases, risk factors for cardiovascular diseases, and clinical examination.
4.1 Demographic characteristics
4.1.1 Age difference in HTN and DM: Our study showed the presence of older age in patients with HTN (alone or with DM) compared with those having DM only. This may be due to the fact that patients with DM develop complications earlier than those with HTN. While Sarkar et al showed that the average age of DM stroke was lower than non-DM [14], a nationally representative study of 1.3 million adults in India showed a high prevalence of HTN (25.3%) and DM (7.5%) in middle and old age, with a higher HTN prevalence among young adults [15].
4.1.2 Age difference in different sexes: Our study demonstrated that in our patients with HTN, DM, there was an increased prevalence of affected men (by both diseases) in the younger population, and increased prevalence of women in the older population (> 75 years old). This may be partially explained by the hormonal changes in women post menopause. Several studies conducted worldwide had comparable results: McDonald et al showed a similar finding in adults with HTN, dyslipidemia, and DM, aged 65 and older (76.6% of women vs 63% of men) [16]. Abramson et al showed that HTN rates were higher in women than men over the age of 65, with a prevalence of 75% in postmenopausal women in the US [17]. Ahmad et al showed also a similar result [18], with an increase in SBP in women secondary to the withdrawal of vasodilator effects of endogenous estrogen, and increased angiotensin II receptor expression [19].
4.2 Clinical examination
4.2.1 Family history between HTN and DM: Family history of previous cardiovascular diseases was present in all groups of our study, and was more common in patients with DM than those with HTN (26.9% vs 15.5%). A national health survey in the US population showed that family history of DM has a significant, independent, and graded association with the prevalence of DM [20]. Another study showed that Indian patients with a positive family history of DM were shown to be more prone to early onset of DM and developing complications [21]. The CARDIA study showed that parental DM was associated with higher fasting blood glucose and insulin levels in all race-sex groups [22]. The CLYDIA study showed that previous ischaemic cerebrovascular disease was more prevalent among Spanish patients with metabolic syndrome, as well as family history of HTN and DM [23]. While many studies had comparable results to ours, many others had opposite results. For instance, a study performed on Sri Lankan adults described a significant higher prevalence of HTN in those with a positive family history [24]. Also, the Malmö Diet and Cancer Study agreed that a fraction of the cardiovascular risk associated with family history is not mediated through DM [25].
4.2.2 Body mass index: There was no statistical difference in BMI between the three different groups in our study. A similar result was found on the Trinidadian population [26] and another one by Tsimihodimos et al [27]. While some studies suggest an increase of prevalence in HTN and DM with higher quintiles of BMI [28], a Malaysian study concluded that waist circumference may be a better indicator for the prediction of obesity-related cardiovascular risk factors in men and women compared to BMI [29].
4.2.3 Low systolic blood pressure in DM mellitus: Patients with DM in our study had lower systolic blood pressure (SBP) levels compared to those with HTN alone, or HTN and DM, with statistically significant differences among these groups. This may be due mainly to the fact that patients with DM only on diagnosis do not have HTN, therefore have a lower SBP. A study by Emdin et al showed that BP lowering in patients with DM was associated with improved mortality [30]. A systematic review proved clear evidence that proportional risk reductions in major cardiovascular disease differed by baseline disease history for DM and chronic kidney disease [31]. Although several studies had comparable results to ours, other studies had different conclusions. A post-hoc analysis of the SPRINT study showed "the lower the better" should not be recommended in patients with DM along with other groups [32].
4.3 Target organ damages
4.3.1 Left ventricular hypertrophy in HTN: Left Ventricular Hypertrophy (LVH) rates were the highest in the HTN group compared to the other groups (DM alone, or HTN and DM). This is mainly due to the remodeling of the left ventricle secondary to hypertensive cardiomyopathy. However, most performed studies worldwide had different findings. A study performed in the rural Chinese population proved that HTN was associated with LVH, and the risk increased when accompanied by DM, especially for women [33]. Another study demonstrated that pre-DM is associated with increased risk of LVH independent of HTN [34]. A Chinese national study demonstrated that tight glycemic and BP control was associated with lower risk of LVH [35]. A trial by Soliman et al proved that reducing SBP <120 mm Hg in patients with HTN and DM produced a great reduction in LVH [36]. Another study by Tanaka et al showed that ECG-LVH parameters were associated with the increased risk of developed cardiovascular events even in the absence of HTN [37].
4.3.2 Proteinuria in HTN and DM: Proteinuria is mainly seen in the hypertensive and diabetic group (36% overall population, 47% in HTN and DM), with statistically significant differences among other groups. This is probably due to the fact that both HTN and DM contribute to this organ damage by renovascular endothelial injury. Albuminuria may be a marker for subclinical cardiovascular damage in DM [38]. Its prevalence was calculated through several studies worldwide: from 6.3% in Luxembourg (HTN and DM associated with 3-4 times higher risks of CKD) [39], and 10.4% in Switzerland [40], to 25.4% in Germany [41], till 58% in Portugal (58% in HTN and DM, 51% in DM, 43% in HTN, 12% in controls) [42], and 67.2% in an outpatient clinics in Afghanistan [43].
A study performed in Bangladesh showed that 45.94% of patients with DM had also HTN, and that microalbuminuria was the first sign of renal involvement [44]. A Korean national survey concluded that albuminuria was associated with the prevalence of HTN in patients with DM [45]. Viazzi et al demonstrated that in patients with DM and HTN, albuminuria is independently associated with stage 3 CKD [46]. Okada et al showed that the prevalence of HTN and DM increased with increasing levels of proteinuria [47]. A study by Wang et al showed statistically significant association between DM and microalbuminuria only in prehypertensive subjects [48].
4.4 Disease recurrence
Disease recurrence, as defined in our study by the number of existing cardiovascular diseases, was more common in patients with HTN and DM, followed by patients with DM, then HTN. This can be explained by the fact that DM is an independent cardiovascular risk factor. Few studies by Wilson et al showed through epidemiological and pathological data that DM is an independent risk factor for CVD in both men and women [49]. Other studies also confirmed the same theory, with a relative risk of cardiovascular mortality of 4.9 in women and 2.1 in men, relative to non-diabetic subjects [50].
4.5 Strength of the study
Our study was a multicentric study where 750 patients were randomly selected, according to their discharge summary diagnosis, and were treated by different specialties: cardiologists and primary care physicians. In addition, the chosen hospitals were in Beirut and Mount Lebanon region and were all university affiliated hospitals, where interns and residents are present, and a continuous medical education program was present, conferences were held routinely, and good documentation and appropriate patient history was taken.
4.6 Weaknesses of the study
Our study was a retrospective as we reviewed the patients’ medical files and collected the data from medical charts; the timing of occurrence of complications was missing for most of the time. In addition, some files had missing data, e.g. none of the patients had his waist circumference documented in medical charts. Weight and height were also missing for some patients. Furthermore, we were not able to assess the social status, the degree of education, and patients’ awareness of their condition.
4.7 Study perspectives
More studies need to be performed for a better understanding of our demographic characteristics: gender study, age correlation. In addition, we need a better system of documentation in hospitals to have a more precise concept of each patient’s background (social, medical...).
5. Conclusion
Our study demonstrated that there was an increased prevalence of affected men (by both diseases) in the younger population, and increased prevalence of women in the older population (> 75 years old). Family history of previous cardiovascular diseases was present in all groups. Disease recurrence, as defined in our study by the number of existing cardiovascular diseases, was more common in patients with both diseases.
Acknowledgements
We are grateful to Mr. Bachir Atallah for his contribution to the statistical analysis of this study. We are extremely grateful for the following hospitals for allowing us to go through their patients’ medical files in order to conduct our study: the academic Beirut Governmental University Hospital, Sacré-Coeur Hospital, Geitawi Hospital, Middle East Institute of Health- Bsalim Hospital, Mount-Lebanon Hospital and Notre Dame du Liban Hospital.
References
- Long NA, Dagogo-Jack S. The Comorbidities of Diabetes and Hypertension: Mechanisms and Approach to Target Organ Protection. J Clin Hypertens (Greenwich) 13 (2011): 244-251.
- Benjamin EJ, Virani SS, Callaway CW. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137 (2018): e67- e492.
- Adib SM, Nuwayhi I, Hamadeh GN. Most common diseases treated in primary health care facilities in Lebanon. J Med Liban 43 (1995): 17-22.
- Milane A, Abdallah J, Kanbar R. Association of hypertension with coronary artery disease onset in the Lebanese population. Springerplus 3 (2014): 533-539.
- Isma'eel H, Shamseddeen W, El Khoury M. Diabetes supersedes dobutamine stress echocardiography in predicting cardiac events in female patients. Clin Exp Obstet Gynecol 37 (2010): 197-200.
- Noubani A, Isma’eel H, Sibai AM. Prevalence, Awareness, and Control of HTN in Greater Beirut Area, Lebanon. International Journal of Hypertension (2018): 1-15.
- Nasrallah MP, Nakhoul NF, Nasreddine L. Prevalence of DM in Greater Beirut area: worsening over time. Endocr Pract 23 (2017): 1091-10100.
- Mancia G, Fagard R, Narkiewicz K. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) 31 (2013): 1281-1357.
- Hyman DJ, Pavlik VN. Characteristics of Patients with Uncontrolled Hypertension in the United States. New England Journal of Medicine Massachusetts Medical Society 345 (2001): 479-486.
- Shaya FT, Yan X, Lin P-J. US Trends in Glycemic Control, Treatment, and Comorbidity Burden in Patients with Diabetes. The Journal of Clinical Hypertension 12 (2010): 826-832.
- Wong K, Glovaci D, Malik S. Comparison of demographic factors and cardiovascular risk factor control among US adults with type 2 diabetes by insulin treatment classification. Journal of diabetes and its Complications 26 (2012): 169-174.
- Alsheikh-Ali AA, Omar MI, Raal FJ. Cardiovascular risk factor burden in Africa and the Middle East: the Africa Middle East Cardiovascular Epidemiological (ACE) study. PLoS One 9 (2014): e102830.
- Tohme RA, Jurjus AR, Estephan A. The prevalence of hypertension and its association with other cardiovascular disease risk factors in a representative sample of the Lebanese population. J Hum Hypertens 19 (2005): 861-868.
- Sarkar RN, Banerjee S, Basu. A Comparative evaluation of diabetic and non-diabetic stroke effect of glycaemia on outcome. J Indian Med Assoc 102 (2004): 551-553.
- Geldsetzer P, Manne-Goehler J, Theilmann M. Diabetes and hypertension in India: A Nationally Representative Study of 1.3 Million Adults. JAMA Intern Med 178 (2018): 363-372.
- McDonald M, Hertz RP, Unger AN. Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among United States adults aged 65 and older. J Gerontol A Biol Sci Med Sci 64 (2009): 256-263.
- Abramson B, Srivaratharajah K, Davis L. Women and Hypertension: Beyond the 2017 Guideline for Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Guidelines_Made_Simple_2017_HBP.pdf
- Ahmad A, Oparil S. Hypertension in Women: Recent Advances and Lingering Questions. Hypertension 70 (2017): 19-26.
- Izumi Y, Matsumoto K, Ozawa Y. Effect of age at menopause on blood pressure in postmenopausal women. Am J Hypertens 20 (2017): 1045-1050.
- Valdez R, Yoon PW, Liu T. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999-2004). Diabetes Care 30 (2007) : 2517-2522.
- Jali MV, Kambar S, Jali SM. Familial early onset of type-2 diabetes mellitus and its complications. N Am J Med Sci 1 (2009): 377-380.
- Burke GL, Savage PJ, Sprafka JM. Relation of risk factor levels in young adulthood to parental history of disease. The CARDIA study. Circulation 84 (1991): 1176-1187.
- Palma Gámiz JL, Conget Donlo I, Bertomeu González V. Prevalence of the metabolic syndrome in Spanish patients with established cardiovascular disease: CLYDIA study. Med Clin (Barc) 128 (2007): 407-413.
- Ranasinghe P, Cooray DN, Jayawardena R. The influence of family history of hypertension on disease prevalence and associated metabolic risk factors among Sri Lankan adults. BMC Public Health 15 (2015): 576-584.
- Fritz J, Shiffman D, Melander O. Metabolic Mediators of the Effects of Family History and Genetic Risk Score on Coronary Heart Disease-Findings from the Malmö Diet and Cancer Study. J Am Heart Assoc 6 (2017): e005254.
- Nayak BS, Sobrian A, Latiff K. The association of age, gender, ethnicity, family history, obesity and hypertension with type 2 diabetes mellitus in Trinidad. Diabetes Metab Syndr 8 (2014) : 91-95.
- Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB. Hypertension and Diabetes Mellitus: Coprediction and Time Trajectories. Hypertension 71 (2018): 422-428.
- Kim YY, Seo E, Baek J. Effects of the Relative Distribution of Body Mass Index on the Incidence of Hypertension and Diabetes Mellitus, Using the Korean Obesity Index. Metab Syndr Relat Disord 17 (2019): 108-114.
- Zaher ZM, Zambari R, Pheng CS. Optimal cut-off levels to define obesity: body mass index and waist circumference, and their relationship to cardiovascular disease, dyslipidaemia, hypertension and diabetes in Malaysia. Asia Pac J Clin Nutr 18 (2009): 209-216.
- Emdin CA, Rahimi K, Neal B. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 313 (2015): 603-615.
- Ettehad D, Emdin CA, Kiran A. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387 (2016): 957-967.
- Hoffmann U. Blood pressure targets: The lower the better does not suit all. Internist (Berl) 59 (2018): 309-315.
- Li T, Chen S, Guo X. Impact of hypertension with or without diabetes on left ventricular remodeling in rural Chinese population: a cross-sectional study. BMC Cardiovasc Disord 17 (2017): 206.
- Rospleszcz S, Schafnitzel A, Koenig W. Association of glycemic status and segmental left ventricular wall thickness in subjects without prior cardiovascular disease: a cross-sectional study. BMC Cardiovasc Disord 18 (2018): 162.
- Zhang W, Liu CY, Ji LN. Blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy in patients with hypertension and diabetes. J Clin Hypertens (Greenwich) 22 (2020): 212-220.
- Soliman EZ, Byington RP, Bigger JT. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients with Diabetes Mellitus: Action to Control Cardiovascular Risk in Diabetes Blood Pressure Trial. Hypertension 66 (2015): 1123-1129.
- Tanaka K, Tanaka F, Onoda T. Prognostic Value of Electrocardiographic Left Ventricular Hypertrophy on Cardiovascular Risk in a Non-Hypertensive Community-Based Population. Am J Hypertens 31 (2018): 895-901.
- Yan S, Yao F, Huang L. Low-grade Albuminuria Associated with Subclinical Left Ventricular Diastolic Dysfunction and Left Ventricular Remodeling. Experimental and Clinical Endocrinology and Diabetes 123 (2015): 515-523.
- Alkerwi A, Sauvageot N, El Bahi I. Prevalence and related risk factors of chronic kidney disease among adults in Luxembourg: evidence from the observation of cardiovascular risk factors (ORISCAV-LUX) study. BMC Nephrol 18 (2017): 358.
- Forni Ogna V, Ogna A, Ponte B. Prevalence and determinants of chronic kidney disease in the Swiss population. Swiss Med Wkly 146 (2016): w14313.
- König M, Gollasch M, Demuth I. Prevalence of Impaired Kidney Function in the German Elderly: Results from the Berlin Aging Study II (BASE-II). Gerontology 63 (2017): 201-209.
- Marques da Silva P, Carvalho D, Nazaré J. Prevalence of microalbuminuria in hypertensive patients with or without type 2 diabetes in a Portuguese primary care setting: The RACE (micRoAlbumin sCreening survEy) study. Rev Port Cardiol 34 (2015): 237-246.
- Shoaib Hamrah M, Hashem Hamrah M, Ishii H. Associations between proteinuria and cardiovascular risk factors among hypertensive patients in Andkhoy, Afghanistan. Nagoya J Med Sci 78 (2016): 377-386.
- Asadujjaman M, Kashem A, Chowdhury AA. Prevalence of Microalbuminuria and Overt Proteinuria in Diabetes Mellitus and their Association with Renal Function. Mymensingh Med J 27 (2018): 467-474.
- Shin KE, Roh YK, Cho KH. The prevalence of hypertension in relation with the normal albuminuria range in type 2 diabetes mellitus within the South Korean population: The Korean National Health and Nutrition Examination Survey, 2011-2012. Prim Care Diabetes 11 (2017): 281-287.
- Viazzi F, Piscitelli P, Giorda C. Association of kidney disease measures with risk of renal function worsening in patients with hypertension and type 2 Diabetes. J Diabetes Complicat 31 (2017): 419-426.
- Okada R, Yasuda Y, Tsushita K. Trace proteinuria by dipstick screening is associated with metabolic syndrome, hypertension, and diabetes. Clin Exp Nephrol 22 (2018): 1387-1394.
- Wang Q, Huang J, Sun Y. Association of microalbuminuria with diabetes is stronger in people with prehypertension compared to those with ideal blood pressure. Nephrology (Carlton) 23 (2018): 690-696.
- Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis 32 (1998): S89-S100.
- Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 100 (1999): 1132-1133.



 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 