Disorders of Phosphate Metabolism: Hypophosphatemia and Hyperphosphatemia
Article Information
Mohammad Tinawi* 
Adjunct Clinical Assistant Professor of Medicine, Indiana University, School of Medicine Northwest-Gary, Nephrology Specialists, Munster, IN, USA
*Corresponding author: Mohammad Tinawi, Nephrology Specialists, P.C., 8840 Calumet Ave, Suite 101, Munster, IN 46321, USA
Received: 28 June 2021; Accepted: 08 July 2021; Published: 17 July 2021
Citation: Mohammad Tinawi. Disorders of Phosphate Metabolism: Hypophosphatemia and Hyperphosphatemia. Archives of Clinical and Biomedical Research 5 (2021): 538-555.
View / Download Pdf Share at FacebookAbstract
Phosphorus in the body exists as phosphate. Phosphate is the most abundant intracellular anion and exists mostly as organic phosphate compounds critical to cellular functions such as adenosine triphosphate (ATP). The kidneys are the main regulator of phosphate homeostasis. There are three hormonal systems responsible for phosphate homeostasis, the parathyroid hormone (PTH), Fibroblast growth factor-23 (FGF-23)/klotho, and 1,25 dihydroxyvitamin D3 (1,25 (OH)2D3, calcitriol). Most cases of hypophosphatemia are acquired and are due to malnutrition as in alcoholism. The kidneys maintain phosphate level in the normal range; therefore, hyperphosphatemia is uncommon until glomerular filtration rate (GFR) falls below 30 ml/min. Hyperphosphatemia is common in patients on renal replacement therapy and is treated with dietary phosphate restriction, and phosphate binders.
Keywords
Hypophosphatemia; Hyperphosphatemia; Electrolyte disorders; Phosphate; Phosphorus
Hypophosphatemia articles; Hyperphosphate-mia articles; Electrolyte disorders articles; Phosphate articles; Phosphorus articles
Article Details
1. Introduction
The focus of this review article is the pathophysiology of phosphate homeostasis in addition to the causes, diagnosis and management of hypophosphatemia and hyperphosphatemia. PubMed database was searched for relevant basic science and clinical articles in addition to the leading journals in nephrology, endocrinology, and internal medicine. The articles reviewed included clinical trials, comprehensive reviews, and case studies deemed of clinical significance. Major textbook chapters were reviewed as well. Phosphorus is a highly reactive element and is never found free in the body. In the human body phosphorus is bound to oxygen in the form of the polyatomic ion phosphate [PO43-]. There are two forms of phosphate in the body, inorganic phosphate (mineral phosphate) and organic phosphate. Phosphate can exist intracellularly or extracellularly [1]. Most of the body phosphate is in the form of organic phosphate complexed with proteins, lipids, and carbohydrates. Phosphate is the most abundant intracellular anion with a concentration of about 100 mmol/l. Most intracellular phosphate is organic (such as creatine phosphate, adenosine phosphate, and erythrocytes 2,3-diphosphoglycerate [2,3-DPG]). Intracellular phosphate is critical to almost all cellular functions. Inorganic phosphate in the cell is sequestered within intracellular organelles and is complexed with other ions such as calcium (Ca) and magnesium (Mg) [2]. Phosphate is essential to cellular structure, enzymatic processes such as glycolysis, and oxidative phosphorylation (formation of ATP).
Serum phosphate assay is the measurement of inorganic phosphate in the serum. The normal range for serum phosphate is 0.8-1.45 mmol/l (2.5-4.5 mg/dl) [3]. Phosphate should not be expressed as mEq/l. To convert from mmol/l to mg/dl multiply by 31 (the atomic weight of phosphorus) and divide by 10 [i.e., multiply by 3.1]. It is important to emphasize that serum (inorganic) phosphate is a very small fraction (0.3%) of total body phosphate. Most of the inorganic phosphate in the serum exists as free phosphate ions (85%), only 10% is protein-bound and 5% is complexed with Ca, Mg or sodium (Na) [4, 5]. Serum inorganic phosphate consists of two forms of orthophosphate, dihydrogen phosphate (H2PO4-) which is a weak acid and monohydrogen phosphate (HPO42-) which is a weak base. At the physiologic pH of 7.40, the HPO42-/H2PO4-ratio is 4:1 [6]. Phosphate intake varies considerably among individuals depending on their intake of protein and phosphate-rich foodstuffs such as dairy products and processed food. Average daily intake of phosphorus is 22.6-64.5 mmol (700-2000 mg). Serum phosphate follows a circadian rhythm with the lowest level around 11 am and the highest level (about 0.19 mmol/l [0.6 mg/dl] higher) in the afternoon [7].
2. Dietary Phosphate
Most foods contain phosphate especially protein-rich sources. Examples are seeds, legumes, fish, meat, and dairy products. Dietary phosphate is derived from animal protein or plant protein. Organic phosphate in animal protein is easily absorbed after hydrolysis. Phosphate in plant protein such as beans and nuts is stored as phytate. Humans do not have the enzyme phytase. Absence of phytase limits the bioavailability of plant-based phosphate to < 50% [8]. Food additives are an important source of inorganic phosphate in the diet. This source is 90% absorbed and is easily overlooked because phosphorus is not usually included in Nutrition Facts labels. This may have significant implications for patients with advanced chronic kidney disease (CKD) in whom phosphate restriction is routinely recommended. Phosphorus is present in over 10% of medication formulations. Some common medications are high in phosphorus such as amlodipine, lisinopril, sitagliptin, and paroxetine [5]. Not all generic formulations have the same amount of phosphorus. The recommended daily allowance (RDA) of phosphorus in adults is 22.6 mmol (700 mg/day of phosphorus which is equivalent to about 2100 mg of phosphate) [9]. Most adults ingest significantly higher amount of phosphorus depending on their protein intake. Patients with CKD are advised to ingest approximately 32 mmol (1000 mg) of phosphorus daily.
3. Phosphate Homeostasis
3.1 Phosphate distribution in the body
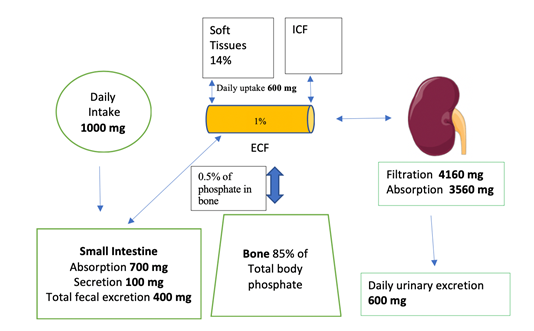
The human body contains approximately 700g of phosphorus (approximately 1% of total body weight) [1]. Most of the organic phosphate in the body (85%) is in the bone. Skeletal phosphate is complexed with Ca as hydroxyapatite [Ca10(PO4)6(OH)2] which gives the bone its mechanical strength. About 14% of phosphate resides in soft tissues, and only 1% is in the extracellular (ECF) space [10]. Only 0.5% of phosphate in the bone is exchanged daily with the ECF. There are two forms of ECF phosphate: organic (70%) and inorganic (30%). Organic phosphate in the ECF exists as phospholipids.
3.2 Intestinal absorption of phosphate
Phosphate absorption occurs in the small intestine, Figure 1. This absorption is mainly regulated by calcitriol (the most active form of vitamin D) and dietary phosphate intake itself. Phosphate absorption occurs transcellularly or paracellularly (passively) [7]. Transcellular absorption is an active process mediated by NaPi2b (sodium-phosphate cotransporter type IIb or Npt2b) [3]. Calcitriol stimulates and nicotinamide (due to reduction in Npt2b expression) inhibits transcellular phosphate absorption. A diet low in phosphate (as recommended in CKD patients) increases intestinal absorption of phosphate due to upregulation of Npt2b-dependant phosphate absorption [11]. The reverse is true. In humans the paracellular absorption of phosphate is more important than the transcellular absorption [12]. Paracellular absorption of phosphate occurs passively through the tight junctions between enterocytes. Tight junctions are composed of claudins. Cations such as aluminum, Ca and Mg bind phosphate in the intestine and decrease its absorption. Certain Ca and Mg salts are used as phosphate binders in patients with CKD.
3.3 Renal handling of phosphate
The kidneys are the main regulator of phosphate homeostasis [7]. Phosphate homeostasis is linked to calcium (Ca) homeostasis [13]. The three hormonal systems that regulate phosphate homeostasis are PTH, calcitriol and FGF-23/klotho.
3.3.1 PTH: PTH decreases renal phosphate absorption resulting in phosphaturia. It exerts this action by acting on the proximal tubule [1]. It has the opposite effect on Ca resulting in an increase in renal Ca absorption and hypocalciuria. PTH stimulates calcitriol synthesis by the kidneys. Moreover, it stimulates the release of both FGF-23 and phosphate by bone cells. It is known that hyperphosphatemia stimulates PTH secretion. Recently Centeno et al. elucidated the underlying mechanism [14]. A rise in phosphate concentration inhibits the activity of the CaSR by non-competitive antagonism. This effect was demonstrated in isolated human parathyroid cells, and it results in an increase in PTH secretion. The crystal structure of the extracellular domain of the CaSR revealed 4 anion binding sites [15]. Phosphate or sulfate (SO4) can occupy these sites resulting in inactivation of the CaSR and stimulation of PTH release. Hyperphosphatemia may block the ability of cinacalcet to activate CaSR and suppress PTH resulting in resistance to the effect of this calcimimetic agent. Therefore, CaSR has binding sites for Ca, Mg and phosphate and it is a calcium and a phosphate sensor [15, 16].
3.3.2 Calcitriol: 1,25(OH)2D3 increases both intestinal and renal absorption of phosphate and Ca. [3]. Calcitriol inhibits PTH production by the parathyroid glands and stimulates FGF-23 production by bone cells.
3.3.3 Fibroblast growth factor-23 (FGF-23)/klotho: FGF-23 is produced in the bone by the osteocytes and osteoblasts [17]. FGF-23 level increases when serum phosphate increases resulting in phosphaturia. FGF-23 decreases phosphate reabsorption in the proximal tubule and the small intestine. FGF-23 requires its cofactor (Klotho) to act on FGF receptor 1 [18]. Both FGF-23 and PTH and are phosphaturic, but they have a contrasting effect on 1α-hydroxylase and subsequently calcitriol (FGF-23 decreases the renal production of calcitriol, while PTH increases it). FGF-23 suppresses the synthesis of PTH in the parathyroid glands, and calcitriol production by the kidneys. FGF-23 level increases early in the course of CKD and this increase has been associated with an increase in all-cause mortality, incident heart failure and cardiovascular events [19]. Table 1. Compares the actions of PTH and FGF-23.
3.3.4 The role of acid-base balance: Phosphate (H2PO4-/HPO42-) is one of the buffer systems in the body that mitigate acid-base disorders [20, 21]. Metabolic acidosis causes phosphaturia which leads to acid removal. Metabolic alkalosis stimulates phosphate reabsorption in the kidney.
3.4 Phosphate reabsorption along the nephron
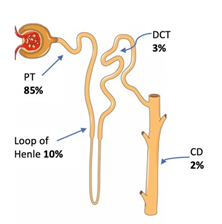
Most of the filtered phosphate is reabsorbed in the proximal tubule (PT) [85%]. The loop of Henle reabsorbs 10%, the distal convoluted tubule (DCT) reabsorbs 3%, and the remaining 2% is reabsorbed in the collecting tubule (CD), Figure 2 [3]. The amount of phosphate excreted in the urine equals the net phosphate uptake by the intestine in a steady state. Phosphate reabsorption is the PT is via the transcellular route and it is an active process that requires movement of Na down its concentration gradient. The apical brush border of the PT contains three different Na-phosphate cotransporters: Npt2a, Npt2c, and PiT-2 (phosphate transporter-2). As mentioned above Npt2b exits in the small intestine. Factors that decrease renal phosphate reabsorption such as high phosphate diet, PTH and FGF-23 downregulate these cotransporters. The reverse is true, factors that increase renal reabsorption of phosphate such as low phosphate diet and calcitriol upregulate these cotransporters [7, 22].
3.5 Phosphate pathophysiology in CKD
In patients with CKD the following sequence of events occurs as the disease progresses: serum Ca decreases due to a decrease in renal calcitriol production. Serum phosphate starts to increase due to the decline in GFR [3]. The decrease in Ca and the increase in phosphate stimulates PTH secretion. The increase in phosphate and PTH stimulates FGF-23 secretion. FGF-23 reduces calcitriol synthesis (by inhibiting renal 1-a hydroxylase and stimulating 24-hydroxylase) and subsequently inhibits intestinal phosphate absorption [17]. The rise in FGF-23 occurs early in the course of CKD (as early as CKD-2) [23]. FGF-23 is independently associated with mortality, initiation of dialysis and cardiovascular events in patients with advanced CKD [24]. Increased FGF-23 levels are also associated with mortality in patients starting hemodialysis [25]. The decrease in Klotho expression in the kidney is an early marker of CKD and chronic kidney disease-mineral and bone disease (CKD-MBD) [26]. Klotho may protect against renal fibrosis and may inhibit vascular calcification. PTH and FGF-23 result in phosphaturia due to a decrease in the number of Na-phosphate cotransporters in the proximal tubule. This will limit the rise in serum phosphate. As renal function worsens, the above mechanism will not be sufficient to mitigate the increase in serum phosphate and hyperphosphatemia ensues. The fractional excretion of phosphate (FE phos) in advanced CKD can surpass 60% [7].
In patients with stage 5 CKD and in patients on renal replacement therapy, it is common to see low or low normal serum Ca, hyperphosphatemia, high PTH, and high FGF-23 levels. These derangements lead to CKD-MBD. Many patients with CKD have secondary hyperparathyroidism due to the rise in PTH resulting from hypocalcemia precipitated by the decrease in calcitriol production. The parathyroid glands become increasingly resistant to FGF-23. The latter will fail to suppress PTH and this will lead to worsening secondary hyperparathyroidism [3]. Secondary hyperparathyroidism is treated with calcitriol or other vitamin D sterols such as doxercalciferol or paricalcitol. Calcimimetics such as cinacalcet and etelcalcetide are effective treatment options for secondary hyperparathyroidism [27].
Figure 1: Phosphate homeostasis. Total phosphorus in an average 70 kg adult is about 700 g. ICF is intracellular fluid. Image of kidney is courtesy of Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License. https://smart.servier.com
|
PTH |
FGF-23 |
|
|
Production site |
Parathyroid gland |
Osteocytes and osteoblasts |
|
Main function |
Ca and phosphate regulations |
Phosphate regulation |
|
Main stimulus |
Hypocalcemia |
Hyperphosphatemia |
|
Renal effect |
Phosphaturia |
Phosphaturia |
|
Effect of calcitriol synthesis |
Increases |
Decreases |
Table 1: PTH and FGF-23 comparison.
Figure 2: Fraction of phosphate reabsorption in different segments of the nephron. Nephron image is courtesy of Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License. https://smart.servier.com Labels added to illustrate the sites of phosphate reabsorption along the nephron.
4. Diagnosis of Phosphate Metabolism Disorders
Diagnosis of hypophosphatemia and hyper-phosphatemia requires a high index of suspicion because phosphate is not included in all routine chemistry panels. Measurement of serum phosphate is easy and available in all laboratories. Other tests are needed including Na, K, Mg and Ca, renal function tests and PTH [28]. Alkaline phosphatase is elevated in mineral and bone diseases such as rickets. FGF-23 assay is only available in reference laboratories. 25-hydroxyvitamin D level is ordered if vitamin D deficiency is suspected. Urine phosphate measurement is needed if urine phosphate wasting is suspected. Normal fractional excretion of phosphate (FE phos) is 5-20%. In other words, the kidneys excrete 5-20% of the total filtered phosphate [7]. In patients with hypophosphatemia FE phos is < 5% unless it is the result of renal phosphate wasting. Renal wasting of phosphate is the excretion of > 3.2 mmol (100 mg) of phosphate daily.
FE phos = 100 x (Urine phos x Serum creatinine) / (Serum phos x Urine creatinine)
5. Hypophosphatemia
5.1 Manifestations
Hypophosphatemia can be mild: serum phosphate 0.5-0.75 mmol/l (1.5-2.4 mg/dl), moderate: serum phosphate 0.32-0.49 mmol/l (1-1.4 mg/dl), or severe: serum phosphate < 0.32 mmol/l (1 mg/dl). Hypophosphatemia is seen in up to 5%-10% of hospitalized patients. The incidence increases to 20%-80% in patients presenting to the emergency department with diabetic ketoacidosis, sepsis and alcohol-related emergencies [29]. Mild hypo-phosphatemia is usually asymptomatic. Moderate hypophosphatemia is associated with metabolic encephalopathy, hemolysis, thrombocytopenia, seizures, poor appetite, myopathy and rhabdom-yolysis [28]. Severe hypophosphatemia can result in respiratory depression, respiratory acidosis, and difficulty in weaning patients off mechanical ventilation [30]. The manifestations of hypophos-phatemia are due to intracellular depletion of ATP and erythrocyte 2,3-DPG [31].
5.2 Causes of hypophosphatemia
Hypophosphatemia results from decreased dietary intake of phosphate, decreased intestinal phosphate absorption, transcellular shift of phosphate, or renal phosphate wasting (increased excretion). Most cases of hypophosphatemia are acquired and are due to malnutrition [28]. Genetic causes are rare. Table 2.
|
Decreased Dietary Intake or Absorption · Malnutrition, starvation, chronic diarrhea, steatorrhea, intestinal malabsorption, and bariatric surgery · Alcoholism and alcohol withdrawal · Vitamin D deficiency · Vitamin D-dependent rickets type IA (VDDR-1A) · Vitamin D-dependent rickets type 1B (VDDR-1B) · Hereditary vitamin D-resistant rickets (HVDRR) |
|
Intracellular Shift · Diabetic ketoacidosis (DKA) and nonketotic hyperglycemia · Total parenteral nutrition (TPN) and refeeding syndrome · Increased insulin secretion · Acute respiratory alkalosis · Cellular uptake of phosphate · Hungry bone syndrome · Cannabinoid hyperemesis syndrome · Salicylate poisoning |
|
Increased Renal Phosphate Excretion · Primary and secondary hyperparathyroidism · Post renal transplantation hypophosphatemia due to high level of PTH and FGF-23 · Diuretics, the diuretic phase of acute tubular necrosis (ATN) and post-obstructive diuresis · Acquired Fanconi syndrome · Tumor-induced osteomalacia (TIO) with high FGF-23 level · Continuous renal replacement therapy (CRRT) |
|
Drug-mediated hypophosphatemia · Corticosteroids, imatinib, carbonic anhydrase inhibitors, theophylline, estrogens, acyclovir, diuretics, phosphate binders, antacids, phenobarbital, phenytoin, IV iron |
|
Genetic Causes of Hypophosphatemia (Familial Hypophosphatemia) Familial Hypophosphatemia Due to FGF-23 Excess · X-Linked hypophosphatemic rickets (XLH) · Autosomal dominant hypophosphatemic rickets (ADHR) · Autosomal recessive hypophosphatemic rickets (ARHR) · McCune-Albright Syndrome (fibrous dysplasia), autosomal dominant, somatic mosaicism FGF-23-Independent Familial Hypophosphatemia · Fanconi Syndrome and type I (proximal renal tubular acidosis [RTA]) · Hereditary hypophosphatemic rickets with hypercalciuria (HHRH), autosomal recessive |
Table 2: Causes of Hypophosphatemia.
5.2.1 Decreased dietary intake or absorption:
5.2.1.1 Malnutrition, chronic diarrhea, steatorrhea, and intestinal malabsorption: malnutrition is a common cause of hypophos-phatemia especially with concomitant poor protein intake and vitamin D deficiency [28]. Fat malabsorption reduces absorption of fat-soluble vitamins such as vitamin D, which results in concomitant hypocalcemia.
5.2.1.2 Alcoholism and alcohol withdrawal: these are common causes of severe hypophosphatemia and are associated with malnutrition and multiple electrolyte disorders [32]. Patients with alcoholism commonly have multiple causes of hypophos-phatemia including poor intake of phosphate and protein, vitamin D deficiency, administration of phosphate-free intravenous fluids (IVF) and hyperventilation [33].
5.2.1.3 Vitamin D deficiency: this is a common cause of hypophosphatemia and is due to inadequate nutrition or lack of sun exposure [28]. Vitamin D deficiency or resistance decreases intestinal phosphate absorption. It also decreases intestinal calcium absorption resulting in hypocalcemia, secondary hyperparathyroidism and subsequently phosphaturia.
5.2.1.4 Vitamin D-dependent rickets type IA (VDDR-1A): VDDR-1A is an autosomal recessive disorder due to an inactivating mutation in theCYP27B1gene that encodes 1α-hydroxylase. Calcitriol level is low with subsequent rise in PTH, hypocalcemia, hypophosphatemia and hyperphos-phaturia [34]. This disorder is treated with calcitriol.
5.2.1.5 Vitamin D-dependent rickets type 1B (VDDR-1B): VDDR-1Bis due to loss-of-function mutation ofCYP2R1, the gene that encodes the enzyme responsible for 25-hydroxylation of vitamin D [35]. It is treated with calcidiol (25-hydroxyvitamin D) and not with nutritional vitamin D compounds such as ergocalciferol and cholecalciferol because they are 25-hydroxyvitamin D precursors.
5.2.1.6 Hereditary vitamin D-resistant rickets (HVDRR): HVDRR is extremely rare and is due to an inactivating mutation in the vitamin D receptor gene [34].
5.2.2 Intracellular shift:
5.2.2.1 Diabetic ketoacidosis (DKA) and nonketotic hyperglycemia: Initially patients manifest with normal or high phosphate (and potassium). After initiation of IVFs and intravenous (IV) insulin, hypophosphatemia (and hypokalemia) ensue due to intracellular shift [7]. Osmotic diuresis and phosphate-free IVFs aggravate hypo-phosphatemia.
5.2.2.2 Total parenteral nutrition (TPN): Insulin in TPN causes intracellular shift of phosphate. Hypophosphatemia will worsen if phosphate is not included in the TPN [7].
5.2.2.3 Increased insulin secretion: Hypophos-phatemia due to increased insulin secretion is seen upon refeeding patients with malnutrition as in anorexia nervosa or alcoholism [36, 37]. Epinephrine and glucagon also drive phosphate intracellularly and can cause mild hypophosphatemia.
5.2.2.4 Acute respiratory alkalosis: as in hyper-ventilation from any cause, mechanical ventilation, sepsis, and salicylate poisoning. This is a common cause of hypophosphatemia in hospitalized patients [36].
5.2.2.5 Cellular uptake of phosphate: as in rapidly proliferating malignancies such as Burkitt’s lymphoma and acute myelogenous leukemia [38].
5.2.2.6 Hungry bone syndrome: this disorder is seen post parathyroidectomy and can result in
hypophosphatemia and severe hypocalcemia [36].
5.2.2.7 Cannabinoid hyperemesis syndrome: Hypophosphatemia probably results from intracellular shift due to hyperventilation [39].
5.2.3 Increased renal phosphate excretion:
5.2.3.1 Primary and secondary hyperparathy-roidism: PTH causes hypercalcemia and hypophosphatemia [40]. The latter is due to its phosphaturic effect.
5.2.3.2 Post renal transplantation hypophos-phatemia: renal phosphate wasting is common post renal transplantation [41]. It is due to elevated FGF-23 level and residual secondary hyperparathyroidism due to end stage renal disease (ESRD).
5.2.3.3 Diuretics, the diuretic phase of acute tubular necrosis (ATN), and post-obstructive diuresis: short-term hypophosphatemia can be seen due to profound diuresis [42].
5.2.3.4 Acquired fanconi syndrome: hypophos-phatemia due to impaired proximal tubule phosphate reabsorption is one of the manifestations of Fanconi syndrome [7]. Some cases are induced by medications, most commonly aminoglycosides, tenofovir, or ifosfamide. A recent report described a case of severe hypophosphatemia due to Fanconi syndrome induced by the checkpoint inhibitor nivolumab [43, 44].
5.2.3.5 Tumor-induced osteomalacia (TIO): Mesenchymal tumors can cause hypophosphatemia due to secretion of FGF-23 [45]. Surgical resection will lead to resolution of hypophosphatemia.
5.2.3.6 Continuous renal replacement therapy (CRRT): hypophosphatemia is common in patients receiving CRRT [46]. Phosphate is routinely added to dialysate or replacement fluids.
5.2.4 Drug-mediated hypophosphatemia:
Multiple mechanisms are at play in this category. Several medications increase phosphate excretion in the urine including corticosteroids, imatinib mesylate, carbonic anhydrase inhibitors such as acetazolamide, theophylline, estrogens, acyclovir, and diuretics such as hydrochlorothiazide [42]. Phosphate binders are used frequently in patients with advanced CKD for the management of hyperphosphatemia. They bind phosphate and decrease its intestinal absorption. Hypophosphatemia is seen if the patient continues to take phosphate binders during periods of poor intake of phosphate-rich foods. Antacids containing alum-inum, calcium, or magnesium can cause hypophos-phatemia by the same mechanism. Phenobarbital and phenytoin can cause hypophosphatemia due to vitamin D deficiency. There has been several case reports of IV iron-induced hypophosphatemia [47]. The proposed mechanisms are elevated FGF-23 and low calcitriol levels in addition to direct proximal tubule toxicity.
5.2.5 Genetic causes of hypophosphatemia (Familial Hypophosphatemia):
Vitamin D dependent and resistant rickets were discussed above. Familial hypophosphatemia is rare. Hypophosphatemia is due to renal phosphate wasting in all of these disorders. Renal phosphate wasting is either FGF-23 dependent or FGF-23 independent.
5.2.5.1 Familial Hypophosphatemia Due to FGF-23 Excess
X-Linked hypophosphatemic rickets (XLH): XLH
is an autosomal dominant disorder responsible for
more than 80% of the cases of familial hypophosphatemia. This form of rickets is due to a loss-of-function mutation in the PHEX gene (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) with subsequent rise in FGF-23 level and renal phosphate wasting [48]. Calcitriol is low or normal in this disorder. Affected children have impaired growth, osteomalacia, rickets, lower extremities pain, and deformities. Burosumab is a recombinant human monoclonal antibody that blocks FGF-23. It was approved by the US Food and Drug Administration (FDA) in 2018 for the treatment of X-linked hypophosphatemia [49].
Autosomal dominant hypophosphatemic rickets (ADHR): ADHR is due to one of several mutations in the FGF-23 gene which makes FGF-23 resistant to cleavage with subsequent rise in FGF-23 level [50].
Autosomal recessive hypophosphatemic rickets (ARHR): ARHR is due to inactivating mutations in the genes for dentin matrix protein 1 (DMP1) or ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) [50].
McCune-Albright syndrome: in this disorder hypophosphatemia is associated with polyostotic (i.e. involving many bones) fibrous dysplasia of the bone [51]. Patients usually have café-au-lait spots on their skin.
5.2.5.2 FGF-23-Independent familial hypophosphatemia
Fanconi syndrome and type I (proximal renal tubular acidosis) RTA: this syndrome manifests with hyperphosphaturia, glucosuria, aminoaciduria, and bicarbonaturia. Renal K wasting is seen as well [50, 52]. Some cases are idiopathic, others are associated with diseases such as Wilson disease, hereditary fructose intolerance, cystinosis, Lowe syndrome, and Dent disease.
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH): HHRH is an autosomal recessive disorder due to mutations in the gene for Npt2c resulting in renal phosphate wasting [53]. FGF-23 is appropriately suppressed in this disorder.
5.3 Treatment
Phosphate is replaced orally (PO) in patients with mild hypophosphatemia. Milk contains 0.9 mg of phosphorus per ml. Commonly 1-2 g is administered daily divided in three doses. Each tablet of K-Phos® Neutral contains 250 mg (8 mmol) of phosphate, 298 mg (13 mmol) of Na and 45 mg (11 mmol) of K. A starting dose is 1-2 tablets 3-4 times daily. Gastrointestinal side effects including diarrhea are the limiting factor. Patients with moderate and severe hypophosphatemia require IV and PO replacement (if feasible) [41]. IV phosphate is available as Na phosphate and K phosphate. The rate should not exceed 7.5 mmol/h. K phosphate should be avoided in patients with hyperkalemia. Serum phosphate is monitored every 12-24 h during IV phosphate replacement.
6. Hyperphosphatemia
Hyperphosphatemia is defined as serum phosphate > 1.45 mmol/l (4.5 mg/dl). Individuals with normal renal function do not develop hyperphosphatemia solely due to increased phosphate intake. Phosphorus intake up to 130 mmol/d (4000 mg/d) will not result in hyperphosphatemia due to the great ability of normal kidneys to increase phosphate excretion. The tolerable upper daily intake level for phosphorus set by the US Food and Nutrition Board is 130 mmol (4000 mg) in individuals 9-70 years of age [9]. Over 50% of patients on renal replacement therapy have hyperphosphatemia despite use of phosphate binders [54].
6.1 Causes of hyperphosphatemia
Common causes of hyperphosphatemia are listed in table 3.
|
Compromised renal function: acute kidney injury and advanced CKD |
|
Cellular release of phosphate: rhabdomyolysis and tumor lysis syndrome |
|
Increased gastrointestinal or renal phosphate absorption: hypoparathyroidism, vitamin D toxicity, acute phosphate nephropathy, fibroblast growth factor receptor inhibitors such as pemigatinib |
|
Phosphate shift to extracellular fluid: lactic acidosis, diabetic ketoacidosis, acute and chronic respiratory acidosis |
|
Genetic: familial tumoral calcinosis |
|
Pseudohyperphosphatemia: hyperlipidemia, liposomal amphotericin B, hyperglobulinemia, hyperbilirubinemia |
Table 3: Causes of Hyperphosphatemia.
6.1.1 Compromised renal function: hyper-phosphatemia is primarily seen in patient with compromised renal function, namely acute kidney injury (AKI) and CKD [55]. The diagnosis is easily made due to concomitant rise in serum creatinine. Chronic hyperphosphatemia is seen in patients with advanced CKD (stage 4 and 5 and in patients on renal replacement therapy). Serum phosphate starts to increase once GFR is < 30 ml/min/1.73 m2 (CKD stage 4 or higher) [56].
6.1.2 Cellular release of phosphate: Acute hyperphosphatemia is seen in patients with cell lysis (such as rhabdomyolysis, malignant hyperthermia, and tumor lysis syndrome [TLS]) due to acute increase in phosphate that overwhelms the excretory ability of the kidneys [57]. Admission hyperphosphatemia is a risk factor for the development of TLS in the appropriate setting [58].
6.1.3 Increased phosphate absorption: as in hypoparathyroidism (increased renal tubular absorption), and vitamin D toxicity (increased GI and renal tubular absorption). Hyperphosphatemia is seen in some patients after taking PO sodium phosphate bowel preparation solution prior to colonoscopy. Acute phosphate nephropathy has been described in some patients taking this bowel preparation manifesting as AKI [59, 60]. Ca phosphate deposits were seen in renal biopsy specimens. These solutions should be avoided in general, particularly in patients with CKD. Pemigatinib is a kinase inhibitor used for the treatment of advanced or metastatic cholan-giocarcinoma with fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement [61]. It results in hyperphosphatemia in over 90% of patients due to FGFR inhibition. Hyperphosphatemia can be severe, and it is due to increased phosphate reabsorption in the proximal tubule. Hyperphos-phatemia is seen with other FGFR inhibitors such as infigratinib.
6.1.4 Phosphate shift to extracellular fluid: as in lactic acidosis and diabetic ketoacidosis (prior to initiation of treatment) [62, 63]. The same effect is seen in acute and chronic respiratory acidosis.
6.1.5 Genetic: Familial tumoral calcinosis is a rare autosomal recessive disorder. Hyperphosphatemia is due to increased proximal tubular reabsorption. Mutations in GALNT3 and FGF-23/Klotho genes have been described leading to a decrease in FGF-23 level, increase in calcitriol level and subsequent hyperphosphatemia [64]. This is the opposite of autosomal dominant and X-linked hypophosphatemic rickets mentioned above.
6.1.6 Pseudohyperphosphatemia: as in hyper-lipidemia, prolonged treatment with liposomal amphotericin B, hyperglobulinemia (especially multiple myeloma), and hyperbilirubinemia [65]. Phosphate level should be analyzed using a different assay.
6.2 Complications of hyperphosphatemia
Most patients are asymptomatic. Some patients develop pruritus. Hyperphosphatemia is associated with vascular calcifications, calciphylaxis (calcific uremic arteriolopathy), and increased morbidity and mortality in CKD patients [66, 67]. Hyperphos-phatemia is potentially associated with increased morbidity and mortality in the general populations as well; however, this issue needs further study [68]. Severe and acute hyperphosphatemia can result in hypocalcemia. The latter can result in muscle cramps, arrhythmias, hypotension, and seizures.
6.3 Treatment of hyperphosphatemia
Acute hyperphosphatemia is treated by addressing the underlying cause such as rhabdomyolysis and TLS. Patients may benefit from IVFs. As in hyperkalemia, IV dextrose and insulin shift phos-phate intracellularly [7]. Acetazolamide increases urinary phosphate excretion. Hemodialysis and CRRT are effective in the management of acute severe hyperphosphatemia seen in the course of AKI as in patients with TLS. Chronic hyperphosphatemia is treated with dietary phosphate restriction and phosphate binders. Dialysis patients are advised to restrict their phosphorus intake to 32 mmol/d (1000 mg/day). If the daily net uptake of phosphate is 60% and dietary compliance is 100%, the weekly phosphorus intake amounts to 135 mmol (4200 mg). A standard 4-hr hemodialysis session removes 22.5-29 mmol (700-900 mg) of phosphorus. If the patient undergoes the customary thrice weekly dialysis, total weekly hemodialysis phosphorus removal is 68-87 mmol (2100-2700 mg) or 50-64% of net phosphorus uptake [5]. In reality this percentage is significantly lower due to non-adherence to low phosphorus diet and phosphate binders. Increasing the frequency and/or the duration of hemodialysis will result in better control of hyperphosphatemia [69]. Peritoneal dialysis (whether continuous ambulatory, automated or continuous cycling) removes approximately 90 ± 32 mmol (2800 ± 1000 mg) of phosphorus weekly [70].
Most patients on renal replacement therapy will need phosphate binders [71]. The role of phosphate binders in patients with CKD who are not on renal replacement therapy remains to be defined. The recently published IMPROVE-CKD trial randomized 278 individuals with stage 3b or 4 CKD and serum phosphate > 1 mmol/l (3.10 mg/dl) to lanthanum carbonate or placebo [72]. After 96 weeks, there was no significant differences between the two groups with regard to arterial stiffness, abdominal aortic calcification, FGF-23, or serum phosphate levels. Phosphate binders limit phosphate intestinal absorption. These binders may upregulate Npt2b in patients with CKD which leads in increased intestinal phosphate absorption in a manner similar to low phosphate diet [11, 73]. This effect may limit the effectiveness of phosphate binders. Commonly used phosphate binders include calcium carbonate, calcium acetate, magnesium carbonate, sevelamer carbonate, sucroferric oxyhydroxide, lanthanum carbonate, and ferric citrate [74]. Non-calcium binders are preferred [61, 62]. Phosphate binders add to the high pill burden in patients on renal replacement therapy. Renal dietitians play a crucial role in the management of hyperphosphatemia in patients on renal replacement therapy [70, 71]. Nicotinamide did not significantly lower FGF-23 or serum phosphate level in a study involving 205 patients with stage 3b/4 CKD over 12 months [79]. Tenapanor is under investigation as a novel treatment for hyperphosphatemia. It is a minimally absorbed inhibitor of sodium hydrogen exchanger 3 (NHE-3). It lowers intestinal phosphate absorption by targeting the paracellular pathway of phosphate absorption. A phase 3 randomized double blind trial in 219 hemodialysis patients showed a mean phosphate reduction of 0.32-0.39 mmol/l (1.0-1.2 mg/dl) over an interval of 8 weeks [80]. The main adverse effect of tenapanor is diarrhea.
7. Conclusion
- There are three main phosphate regulating hormonal systems: PTH, vitamin D, and FGF-23/klotho.
- Phosphate level is maintained by the interplay between the above hormones and the bowel (phosphate absorption), the kidneys (phosphate reabsorption and excretion), phosphate shift between ICF and ECF, and bone (phosphate uptake and release).
- Hypophosphatemia is the result of poor dietary intake, decreased intestinal phosphate absorption, renal phosphate wasting, or intracellular shift of phosphate.
- The most common causes of hypophos-phatemia are malnutrition and vitamin D deficiency.
- Hyperphosphatemia is mainly seen in patients
with AKI, stage 4 or 5 CKD, and patients on renal replacement therapy. It is treated with dietary phosphate restriction and phosphate binders.
References
- Penido MGMG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol 27 (2012): 2039-2048.
- Prasad N, Bhadauria D. Renal phosphate handling: Physiology. Indian J Endocrinol Metab 17 (2013): 620-627.
- Blaine J, Chonchol M, Levi M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin J Am Soc Nephrol 10 (2015): 1257-1272.
- Bansal V. Serum Inogranic Phosphorus. In: Walker H, Hall W, Hurst J (eds) Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworths (1990): 895-899.
- Anjay Rastogi, Nisha Bhatt, Sandro Rossetti, Judith Beto. Management of Hyperphosphatemia in End-Stage Renal Disease: A New Paradigm. J Ren Nutr (2020): 1-14.
- Chonchol M, Smogorzewski M, Stubbs J. Disorders of Calcium, Magnesium, and Phosphate Balance. In: Yu ASL, Chertow GM, Luyckx V, et al. (eds) Brenner & Rector’s The Kidney. Philadelphia: Elsevier Inc (2020): 580-613.
- Kestenbaum B, Houillier P. Disorders of Calcium, Phosphate, and Magnesium Metabolism. In: Johnson R, Feehally J, Floege J, et al. (eds) Comprehensive Clinical Nephrology (2018): 124-141.
- Kamyar Kalantar-Zadeh, Lisa Gutekunst, Rajnish Mehrotra, Csaba P Kovesdy, Rachelle Bross, Christian S Shinaberger, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 5 (2010): 519-530.
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Epub ahead of print (2006).
- Chang AR, Anderson C. Dietary Phosphorus Intake and the Kidney. Annu Rev Nutr 37 (2017): 321-346.
- Tamara Isakova, Joachim H Ix, Stuart M Sprague, Kalani L Raphael, Linda Fried, Jennifer J Gassman, et al. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 26 (2015): 2328-2339.
- Saurette M, Alexander RT. Intestinal phosphate absorption: The paracellular pathway predominates?. Exp Biol Med 244 (2019): 646-654.
- Tinawi M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus 13 (2021): e12420.
- Patricia P Centeno, Amanda Herberger, Hee-Chang Mun, Chialing Tu, Edward F Nemeth, Wenhan Chang, et al. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun 10 (2019): 1-12.
- Yong Geng, Lidia Mosyak, Igor Kurinov, Hao Zuo, Emmanuel Sturchler, Tat Cheung Cheng, et al. Structural mechanism of ligand activation in human calcium-sensing receptor. Elife 5 (2016): e13662.
- Jensen A, Brauner-Osborne H. Allosteric Modulation of the Calcium-Sensing Recep-tor. Curr Neuropharmacol 5 (2007): 180-186.
- Lederer E. Regulation of serum phosphate. J Physiol 592 (2014): 3985-3995.
- Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 69 (2007): 341-359.
- Joachim H Ix, Ronit Katz, Bryan R Kestenbaum, Ian H de Boer, Michel Chonchol, Kenneth J Mukamal, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60 (2012): 200-207.
- Lee Hamm L, Nakhoul N, Hering-Smith KS. Acid-base homeostasis. Clin J Am Soc Nephrol 10 (2015): 2232-2242.
- Tinawi M. Pathophysiology, Evaluation and Management of Metabolic Acidosis. Arch Clin Biomed Res 5 (2021): 85-109.
- Lederer E. Renal phosphate transporters. Curr Opin Nephrol Hypertens 23 (2014): 502-506.
- Wolf M. Forging Forward with 10 Burning Questions on FGF23 in Kidney Disease. J Am Soc Nephrol 21 (2010): 1427-1435.
- Jessica Kendrick, Alfred K Cheung, James S Kaufman, Tom Greene, William L Roberts, Gerard Smits, et al. FGF-23 Associates with Death, Cardiovascular Events, and Initiation of Chronic Dialysis. J Am Soc Nephrol 22 (2011): 1913-1922.
- Orlando M Gutiérrez, Michael Mannstadt, Tamara Isakova, Jose Alejandro Rauh-Hain, Hector Tamez, Anand Shah, et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N Engl J Med 359 (2008): 584-592.
- Kuro-O M. Phosphate and Klotho. Kidney Int Suppl 79 (2011): S20-S23.
- Friedl C, Zitt E. Role of etelcalcetide in the management of secondary hyperpara-thyroidism in hemodialysis patients: a review on current data and place in therapy. Drug Des Devel Ther 12 (2018): 1589-1598.
- Imel EA, Econs MJ. Approach to the hypophosphatemic patient. J Clin Endocrinol Metab 97 (2012): 696-706.
- Miller DW, Slovis CM. Hypophosphatemia in the emergency department therapeutics. Am J Emerg Med 18 (2000): 457-461.
- Tinawi M. Respiratory Acid-Base Disorders: Respiratory Acidosis and Respiratory Alkalosis. Arch Clin Biomed Res 5 (2021): 158-168.
- Lichtman MA, Miller DR, Cohen J, Waterhouse C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med 74 (1971): 562-568.
- Rigas Kalaitzidis. Metabolic Abnormalities in Alcoholic Patients: Focus on Acid Base and Electrolyte Disorders. J Alcohol Drug Depend 3 (2015): 1000185.
- Tinawi M. New Trends in the Utilization of Intravenous Fluids. Cureus 13 (2021): e14619.
- Malloy PJ, Feldman D. Genetic disorders and defects in vitamin D action. Endocrinol Metab Clin North Am 39 (2010): 333-346.
- Arnaud Molin, Arnaud Wiedemann, Nick Demers, Martin Kaufmann, Jérémy Do Cao, Laurent Mainard, et al. Vitamin D–Dependent Rickets Type 1B (25-Hydroxylase Deficiency): A Rare Condition or a Misdiagnosed Condition?. J Bone Miner Res 32 (2017): 1893-1899.
- Manghat P, Sodi R, Swaminathan R. Phosphate homeostasis and disorders. Ann Clin Biochem 51 (2014): 631-656.
- O’Connor G, Nicholls D. Refeeding hypophosphatemia in adolescents with anorexia nervosa: A systematic review. Nutr Clin Pract 28 (2013): 358-364.
- Wollner A, Shalit M, Brezis M. Tumor genesis syndrome. Hypophosphatemia accompanying Burkitt’s lymphoma cell leukemia. Miner Electrolyte Metab 12 (1986): 173-175.
- Cadman PE. Hypophosphatemia in Users of Cannabis. Am J Kidney Dis 69 (2017): 152-155.
- Felsenfeld AJ, Rodriguez M. Phosphorus, regulation of plasma calcium, and secondary hyperparathyroidism: A hypothesis to integrate a historical and modern perspective. J Am Soc Nephrol 10 (1999): 878-890.
- Brunelli S, Goldfarb S. Hypophosphatemia: Clinical Consequences and Management. J Am Soc Nephrol 18 (2007): 1999-2003.
- Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: A review. QJM 103 (2010): 449-459.
- Tinawi M, Bastani B. A case of Fanconi syndrome as a complication of treatment with a checkpoint inhibitor in a patient with hepatocellular carcinoma. J Nephropathol 9 (2020): e19.
- Tinawi M, Bastani B. Nephrotoxicity of Immune Checkpoint Inhibitors: Acute Kidney Injury and Beyond. Cureus 12 (2020): e12204.
- Juan Feng, Yan Jiang, Ou Wang, Mei Li, Xiaoping Xing, Li Huo, et al. The diagnostic dilemma of tumor induced osteomalacia: A retrospective analysis of 144 cases. Endocr J 64 (2017): 675-683.
- Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G. Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand 55 (2011): 39-45.
- Nataatmadja MS, Francis R. Recurrent severe hypophosphatemia following intravenous iron administration. Clin Case Reports 8 (2020): 243-246.
- Prié D, Friedlander G. Genetic disorders of renal phosphate transport. N Engl J Med 362 (2010): 2399-2409.
- Thomas O. Carpenter, Michael P Whyte, Erik A Imel, Annemieke M Boot, Wolfgang Högler, Agnès Linglart, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 378 (2018): 1987-1998.
- Lee JY, Imel EA. The changing face of hypophosphatemic disorders in the FGF-23 era. Pediatr Endocrinol Rev 10 (2013): 367-379.
- Robinson C, Collins MT, Boyce AM. Fibrous Dysplasia/McCune-Albright Syndrome: Clinical and Translational Perspectives. Curr Osteoporos Rep 14 (2016): 178-186.
- Daniella Magen, Liron Berger, Michael J Coady, Anat Ilivitzki, Daniela Militianu, Martin Tieder, et al. A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med (2010): 1102-1109.
- Bettina Lorenz-Depiereux, Anna Benet-Pages, Gertrud Eckstein, Yardena Tenenbaum-Rakover, Janine Wagenstaller, Dov Tiosano, et al. Hereditary hypophosphatemic rickets with hyper-calciuria is caused by mutations in the sodium-phosphate co-transporter gene SLC34A3. Am J Hum Genet 78 (2006): 193-201.
- Covic A, Rastogi A. Hyperphosphatemia in patients with ESRD: Assessing the current evidence linking outcomes with treatment adherence. BMC Nephrol 14 (2013): 153.
- Hruska KA, Mathew S, Lund R, et al. Hyperphosphatemia of chronic kidney disease. Kidney Int 74 (2008): 148-157.
- Bryan Kestenbaum, Joshua N Sampson, Kyle D Rudser, Donald J Patterson, Stephen L Seliger, Bessie Young, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16 (2005): 520-528.
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 361 (2009): 62-72.
- Michael Darmon, François Vincent, Laurent Camous, Emmanuel Canet, Caroline Bonmati, Thorsten Braun, et al. Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique. Br J Haematol 162 (2013): 489-497.
- Hoffmanová I, Kraml P, Andel M. Renal risk associated with sodium phosphate medication: Safe in healthy individuals, potentially dangerous in others. Expert Opin Drug Saf 14 (2015): 1097-1110.
- Monica Schaefer, Emily Littrell, Amina Khan, Mark E Patterson. Estimated GFR decline following sodium phosphate enemas versus polyethylene glycol for screening colonoscopy: A retrospective cohort study. Am J Kidney Dis 67 (2016): 609-616.
- Ghassan K Abou-Alfa, Vaibhav Sahai, Antoine Hollebecque, Gina Vaccaro, Davide Melisi, Raed Al-Rajabi, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 21 (2020): 671-684.
- O’connor LR, Klein KL, Bethune JE. Hyperphosphatemia in Lactic Acidosis. N Engl J Med 297 (1977): 707-709.
- Kebler R, McDonald FD, Cadnapaphornchai P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med 79 (1985): 571-576.
- Sprecher E. Familial tumoral calcinosis: From characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol 3 (2010): 652-660.
- Valentina Molinaris, Mario G Bianchetti, Gregorio P Milani, Sebastiano AG Lava, Roberto Della Bruna, Giacomo D Simonetti, et al. Interferences in the measurement of circulating phosphate: A literature review. Clin Chem Lab Med. Epub ahead of print (2020).
- Block GA, Hulbert-Shearon TE, Levin NW, Port F K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31 (1998): 607-617.
- Askar AM. Hyperphosphatemia: The hidden killer in chronic kidney disease. Saudi Med J 36 (2015): 13-19.
- Chang AR, Grams ME. Serum Phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): Effect modification by fasting. Am J Kidney Dis 64 (2014): 567-573.
- John T Daugirdas, Glenn M Chertow, Brett Larive, Andreas Pierratos, Tom Greene, Juan Carlos Ayus, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 23 (2012): 727-738.
- Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y. Superior dialytic clearance of beta(2)-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int 70 (2006): 794-799.
- Tonelli M, Pannu N, Manns B. Oral Phosphate Binders in Patients with Kidney Failure. N Engl J Med 362 (2010): 1312-1324.
- Nigel D Toussaint, Eugenia Pedagogos, Nicole M Lioufas, Grahame J Elder, Elaine M Pascoe, Sunil V Badve, et al. A Randomized Trial on the Effect of Phosphate Reduction on Vascular End Points in CKD (IMPROVE-CKD). J Am Soc Nephrol 31 (2020): ASN.2020040411.
- Radanovic T, Wagner CA, Murer H, Jürg Biber. Regulation of Intestinal Phosphate Transport I. Segmental expression and adaptation to low-Pi diet of the type IIb Na+-P i cotransporter in mouse small intestine. Am J Physiol - Gastrointest Liver Physiol 288 (2005): G496-G500.
- Nigar Sekercioglu, Lehana Thabane, Juan Pablo Díaz Martínez, Gihad Nesrallah, Christopher J Longo, Jason W Busse, et al. Comparative effectiveness of phosphate binders in patients with chronic kidney disease: A systematic review and network meta-analysis. PLoS One 11 (2016): e0156891.
- Francesco Locatelli, Lucia Del Vecchio, Leano Violo, Giuseppe Pontoriero. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: A comparison of safety profiles. Expert Opin Drug Saf 13 (2014): 551-561.
- Moe SM, Chertow GM. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol 1 (2006): 697-703.
- Martin CJ, Reams SM. The renal dietitian’s role in managing hyperphosphatemia and secondary hyperparathyroidism in dialysis patients: A national survey. J Ren Nutr 13 (2003): 133-136.
- Kawate Y, Miyata H. The importance of nutritional intervention by dietitians for hyperphosphatemia in maintained hemodialysis patients. Ren Replace Ther 3 (2017): 19.
- Joachim H Ix, Tamara Isakova, Brett Larive, Kalani L Raphael, Dominic S Raj, Alfred K Cheung, et al. Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: The COMBINE trial. J Am Soc Nephrol 30 (2019): 1096-1108.
- Geoffrey A Block, David P Rosenbaum, Andrew Yan, Glenn M Chertow. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: A randomized phase 3 trial. J Am Soc Nephrol 30 (2019): 641-652.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 