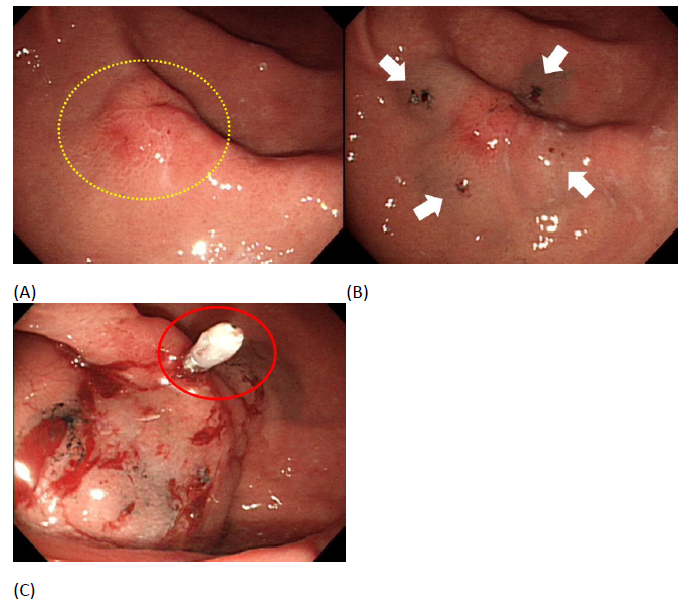
A Valuable Endoscopic Tattooing Method for Early Gastric Cancer Localization before Laparoscopic Surgery
Article Information
Sheng-Fu Wang1,2, Chi-Huan Wu1,2, Jun-Te Hsu2,3, Mu-Hsien Lee1,2, Cheng-Hui Lin1,2, Ta-Sen Yeh2,3, Chun-Jung Lin1,2, Kai-Feng Sung1,2*
1Department of Gastroenterology and Hepatology, Chang-Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
2School of Medicine, College of Medicine, Chang-Gung University, Taoyuan, Taiwan
3Department of General Surgery, Chang-Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan
|
Total |
With clip |
Without clip |
p value |
|
|
(N=78) |
(N=15) |
(N=63) |
||
|
Age |
59.5±13.4 |
56.4±14.6 |
60.2±13.4 |
0.611 |
|
Gender |
0.345 |
|||
|
Male |
30(38.5%) |
3(20%) |
27(42.9%) |
|
|
Female |
48(61.5%) |
12(80%) |
36(57.1%) |
|
|
Tumor location by endoscopy |
0.58 |
|||
|
High-body |
6(7.7%) |
0(0%) |
6(9.5%) |
|
|
Mid-body |
24(30.8%) |
6(40%) |
18(28.6%) |
|
|
Low-body |
30(38.5%) |
3(20%) |
27(42.9%) |
|
|
Antrum |
18(23.1%) |
6(40%) |
12(19.0%) |
|
|
Time to surgery (hour) |
8.3±11.7 |
6.9±10.2 |
8.7±12.3 |
0.76 |
|
Endoscopic finding |
||||
|
Duration of procedure (minutes) |
11.7±5.6 |
12.6±4.2 |
11.5±6.0 |
0.71 |
|
Tumor size |
2.0±1.3 |
1.8±0.8 |
2.1±1.4 |
0.369 |
|
Superficial ulcer |
0.345 |
|||
|
Yes |
30(38.5%) |
3(20%) |
27(42.9%) |
|
|
No |
48(61.5%) |
12(80%) |
36(57.1%) |
|
|
Paris type |
0.687 |
|||
|
0-Is |
6(7.7%) |
0(0%) |
6(9.5%) |
|
|
0-Ip |
0(0.0%) |
0(0%) |
0(0%) |
|
|
0-IIa |
0(0.0%) |
0(0%) |
0(0%) |
|
|
0-IIb |
24(30.8%) |
3(20%) |
21(33.3%) |
|
|
0-IIc |
45(57.7%) |
12(80%) |
33(52.4%) |
|
|
0-III |
3(3.8%) |
0(0%) |
3(4.8%) |
|
|
Type of surgery |
0.85 |
|||
|
Subtotal gastrectomy |
63(80.8%) |
12(80%) |
51(81.0%) |
|
|
Total gastrectomy |
3(3.8%) |
0(0%) |
3(4.8%) |
|
|
Pylorus preserving segmental gastrectomy |
12(15.4%) |
3(20%) |
9(14.3%) |
|
|
Adjuvant chemotherapy |
0.473 |
|||
|
Yes |
6(7.7%) |
0(0%) |
6(9.5%) |
|
|
No |
72(92.3%) |
15(100%) |
57(90.5%) |
Table 1: Patient characteristics
|
With clip |
Without clip |
p value |
|
|
(N=15) |
(N=63) |
||
|
T stage |
0.761 |
||
|
T1a |
12(80%) |
36(57.1%) |
|
|
T1b |
3(20%) |
18(28.6%) |
|
|
T2 |
0(0%) |
6(9.5%) |
|
|
T3 |
0(0%) |
3(4.8%) |
|
|
Lauren class |
0.619 |
||
|
Intestinal type |
6(40%) |
18(28.6%) |
|
|
Diffuse type |
9(60%) |
45(71.4%) |
|
|
Distance from margin |
3.0±1.5 |
3.2±2.0 |
0.798 |
|
Sufficient margin[1] |
0.856 |
||
|
Yes |
12(80%) |
48(76.2%) |
|
|
No |
3(20%) |
15(23.8%) |
|
|
[1] T1: distance from margin ≥ 2 cm, T2~T4: distance from margin ≥ 3 cm |
|||
Table 2: Pathologic features.
|
With clip |
Without clip |
p value |
|
|
(N=15) |
(N=63) |
||
|
Duration of surgery (minute) |
286±46.7 |
322.4±101.6 |
0.447 |
|
Blood loss (mL) |
60±38.1 |
56.2±105.8 |
0.938 |
|
Hospital days |
11.6±1.8 |
16.0±24.0 |
0.687 |
|
Time to intake (day) |
6.6±1.9 |
9.6±13.1 |
0.623 |
|
complication |
0.915 |
||
|
No |
12(80%) |
45(71.4%) |
|
|
Fever |
3(20%) |
12(19%) |
|
|
Wound infection |
0(0%) |
3(4.8%) |
|
|
Internal bleeding |
0(0%) |
3(4.8%) |
|
|
Recurrence |
1 |
||
|
Yes |
0(0%) |
0(0%) |
|
|
No |
15(100%) |
63(100%) |
Table 3: Surgical outcome.
|
Pylorus preserving segmentectomy |
Subtotal gastrectomy |
p value |
|
|
(N=12) |
(N=63) |
||
|
Duration of surgery (minute) |
225±30.0 |
326.2±90.7 |
0.040* |
|
Blood loss (mL) |
37.5±41.4 |
61.0±105.5 |
0.67 |
|
Hospital days |
13.3±5.0 |
15.5±24.0 |
0.858 |
|
Time to intake (day) |
9.3±4.3 |
8.9±13.1 |
0.965 |
|
complication |
0.236 |
||
|
No |
6(50%) |
51(81%) |
|
|
Fever |
6(50%) |
6(9.5%) |
|
|
Wound infection |
0(0%) |
3(4.8%) |
|
|
Internal bleeding |
0(0%) |
3(4.8%) |
|
|
Recurrence |
1 |
||
|
Yes |
0(0%) |
0(0%) |
|
|
No |
12(100%) |
63(100%) |
|
|
Specimen |
|||
|
Distance from margin |
3.1±2.9 |
3.1±1.6 |
0.986 |
|
Stomach diameter |
11.2±3.8 |
12.7±4.4 |
0.52 |
|
Tumor diameter |
1.3±0.5 |
2.1±1.7 |
0.37 |
|
Tumor/stomach ratio |
0.12±0.06 |
0.19±0.18 |
0.451 |
|
Sufficient margin[1] |
0.184 |
||
|
Yes |
6(50%) |
51(81%) |
|
|
No |
6(50%) |
12(19%) |
|
|
[1] T1: distance from margin ≥2 cm, T2~T4: distance from margin ≥3 cm |
|||
Table 4: Different type of surgery.
|
Sufficient margin |
Insufficient margin |
p value |
|
|
(N=60) |
(N=18) |
||
|
Endoscopy finding |
|||
|
Tumor size under endoscopy |
1.8±1.1 |
2.9±1.7 |
0.06 |
|
Tumor size>2 cm |
24(40%) |
12(66.7%) |
0.25 |
|
Tumor size<2 cm |
36(60%) |
6(33.3%) |
|
|
Superficial ulcer |
0.211 |
||
|
Yes |
27(45%) |
3(16.7%) |
|
|
No |
33(55%) |
15(83.3%) |
|
|
Paris type |
0.061 |
||
|
0-Is |
3(5%) |
3(16.7%) |
|
|
0-Ip |
0(0%) |
0(0%) |
|
|
0-IIa |
0(0%) |
0(0%) |
|
|
0-IIb |
15(25%) |
9(50%) |
|
|
0-IIc |
42(70%) |
3(16.7%) |
|
|
0-III |
0(0%) |
3(16.7%) |
|
|
Pathologic features |
|||
|
Tumor size |
1.4±0.7 |
3.4±2.7 |
0.005* |
|
Tumor size>2 cm |
9(15%) |
12(66.7%) |
0.012* |
|
Tumor size<2 cm |
51(85%) |
6(33.3%) |
|
|
T stage |
0.693 |
||
|
T1a |
36(60%) |
12(66.7%) |
|
|
T1b |
18(30%) |
3(16.7%) |
|
|
T2 |
3(5%) |
3(16.7%) |
|
|
T3 |
3(5%) |
0(0%) |
|
|
Lauren class |
0.877 |
||
|
Intestinal type |
18(30%) |
6(33.3%) |
|
|
Diffuse type |
42(70%) |
12(66.7%) |
|
|
*p value<0.05 considered as clinical significance |
|||
Table 5: Factors influence sufficient negative margin.
4. Discussion
Based on our results, all the variables had no clinical significance between tattooing with or without metal clips. All patients had margin free resection. 76% patients (48/63) had adequate safety margins even without the use of clips. This validates that our endoscopic tattooing method is efficient enough for tumor localization preoperatively even without clips used. We also speculate that our endoscopic tattooing method may be able to facilitate laparoscopic surgery with pylorus preserving segmentectomy as there is no clinical significance in perioperative outcome, distance from margin, and proportion of adequate margin achieved between the two groups. However, interpretation was careful due to limited number of patients and the lower proportion of adequate margin achieved in pylorus preserving segmentectomy compared to subtotal gastrectomy (50% vs. 81%) though no clinical significance was found. Furthermore, 15 patients had metal clips but none of them used intraoperative fluoroscopy due to negative resection margins by frozen section or by inspection from surgeon. This showed that the use of metal clip is not required for localization in early gastric cancer and does not depend on the type of laparoscopic surgery performed. It is also cost-effective for patients because of fees from metal clips during endoscopy. Safety is the most important issue in this invasive procedure. 21 patients had complications that include fever, wound infection and internal bleeding after the surgery though none are related to endoscopic tattooing. Previous studies had reported the most common and disturbing adverse effect after the tattooing being peritoneal staining, when unintended transmural injection occurs which may induce peritonitis and could obscure the surgical dissection planes, making surgery more dangerous and challenging [27, 28]. No obvious dye spillage was found during the operations in our study. However, India ink, mainly for colonic lesions, was used in previous studies, which is different to our method in the stomach [29, 30]. We used SPOT as the dye for tattooing, which is similar to India ink but has less inflammatory effects and is the only U.S. Food and Drug Administration (FDA) certified product for tattooing [25]. Another possible reason we had no adverse effects is that the gastric wall is thicker than the colon’s thus reducing the chance of transmural injection. We also avoided dye dilution by saline and pre-injection with saline, which is the submucosal injection method used in previous studies [17]. Due to geographic variation in gastric cancer’s incidence, different approaches are noted in different countries. More than 70% of cases occur in developing countries including Central and Eastern Europe, South America and Eastern Asia, mainly in China [31]. Gastric cancer in western countries are more advanced with locations more proximal and with a higher proportion of diffuse type histologically. After debating for decades about treatment and surveillance protocol between East and West countries, there is recent consensus in treating gastric cancers based on two systems of guidelines which include the Japanese Gastric Cancer Association (JGCA) and the Union for International Cancer Control (UICC/TNM) [3, 32-34]. Increased detection in earlier stages of the disease, which results in better prognosis, is attributable to extensive screening with esophagogastrodudenoscopy (EGD) done in Japan due to the high incidence of gastric cancer. Therefore, laparoscopic surgery for early gastric cancers developed rapidly in recent years in Japan and Korea but much slower in Western countries [35, 36]. However, preoperative localization is usually needed due to the lack of tactile feedback during surgery. Recently, Yamazaki et al reported a study using metal clips along with tattooing with India ink for early gastric cancer localization. They applied two metal clips over the oral site of the lesions, injected 2 mL of normal saline into the submucosal layer, 0.1 mL of India ink, and 1 mL of saline injected near the clips. Additionally, some cases had biopsy performed to ensure negative margins in their study. Although all patients had R0 resection, 11.1% patients had widespread stains [24]. Different from their practice, we only injected 0.1 mL of SPOT directly without dilution at four quadrants of the target lesion and no cases had peritoneal staining intraoperatively. In addition, we did not do any negative biopsy during endoscopic tattooing and all patients had negative resection margins. Another study conducted by Kim et al used two or three metal clips proximal to the tumor for preoperative localization [21]. All patients in their study had margin free resection with a mean proximal margin length of 3.42 ± 2.02 cm, which is similar to our patients that underwent endoscopic tattooing without clips (mean distance from margin: 3.2±2.0 cm). However, C-arm fluoroscopy should be used to localize the metal clip in the operation room and the extra radiation would be exposed to the surgeon.
According to our study, 80% of patients had sufficient safety margin resections from the tumor whether clips were used. In other words, around 20% patients may have had a risk of recurrence. We also found that 50% of patients had insufficient safety margins in the group of pylorus preserving gastrectomy. Thus we would like to figure out whether there are any risk factors that could predict insufficient safety margins during endoscopic tattooing. Previous study mentioned that surgeons can only decide the resection margin depending on the marking the endoscopist located preoperatively, especially in early gastric cancer [21]. It implied that the location we marked at from the tumor is important although the surgeon may resect at least 2cm distant from the tattooing for safety margin. However, this could be affected by submucosal spreading or the histological feature of poorly differentiated adenocarcinoma. Kumazu et al. enrolled 2757 patients that underwent gastrectomy for gastric cancer and evaluated risk factors of microscopic positive resection margins [37]. They found that remnant gastric cancer (odds ratio [OR] 4.7), esophageal invasion (OR 6.3), tumor size ≥80 mm (OR 3.9), and a histopathological diagnosis of undifferentiated type (OR 3.6), macroscopic type 4 (OR 3.7), or pT4 disease (OR 4.6) had correlation to R1 resection. We also analyzed these possible risk factors and only tumor size in specimen had clinical significance, particularly tumor size above 2 cm. In addition, previous studies also showed a tumor size>2 cm implied a more advanced stage [5]. Regarding the tumor size under endoscopy, we can only observe the trend that larger size under endoscopy is more likely to have insufficient safety margin. One third of tumors with sizes more than 2 cm had insufficient resection margin, compared to 14% in tumor sizes less than 2 cm. However, no clinical significance was found under statistical analysis. Therefore, we suggest that the location of tattooing from the tumor edge or the transection line from the center of the tattoo could be more distant if the size of tumor is more than 2 cm under endoscopy or CT image.
5. Conclusion
Our novel method with SPOT at four-quadrants in early gastric cancer before laparoscopic subtotal gastrectomy is a feasible and safe method even without metal clips used. It is feasible in pylorus preserving segmentectomy as no statistical difference was found in surgical outcomes and pathological features compared to subtotal gastrectomy with limited patients in our study. 18(23%) patients had insufficient safety margins although no tumor recurrence was found when followed up (for how long in this 6 patients group). In addition, our analysis showed that tumor size, particularly above 2 cm, is a risk factor for insufficient safety margins using this method of endoscopic tattooing localization. Thus the markings could be located more distant from the tumor’s edge or the surgeon could modify the resection margin in high risk patients. There are some limitations with our study. First, this is a retrospective and single-centered study. Second, the limited number of patients may cause outcomes to not be as convincible. Third, EUS features could not be evaluated as only part of patients had done EUS preoperatively.
Declaration
Ethical Approval and Consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Chang Gung Medical Foundation Institutional Review Board (protocol code 202101143B0 and approval on 2021/7/5). Consent was obtained from all subjects involved in the study.
Consent for publication
not required
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Competing interests
None of the authors of this study has any financial interest or conflict with industries or parties
Funding
This research received no external funding.
Authors' contributions
Conceptualization, S.-F.W. and K.-F.S.; methodology, K.-F.S. and C.-H.L.; software, J.-T.H.; validation, J.-T.H., C.-H.W. and M.-H.L.; formal analysis, S.-F.W.; investigation, C.-J.L.; resources, K.-F.S.; data curation, S.-F.W.; writing—original draft preparation, S.-F.W.; writing—review and editing, K.-F.S.; visualization, C.-J.L.; supervision, K.-F.S. and T.-S.Y.; project administration, S.-F.W.; funding acquisition, S.-F.W. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We thank the team of endoscopic center in Chang Gung Memorial Hospital to facilitate this work. We also thank the surgeons of general surgery in Chang Gung Memorial Hospital to give advisement about the part of operation in this article.
References
- Ferlay J, Isabelle S, Rajesh D, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136 (2015): 359-386.
- Smyth EC, Magnus L, Heike IG, et al. Gastric cancer. Lancet 396 (2020): 635-648.
- Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20 (2017): 1-19.
- Murakami T. Early cancer of the stomach. World Journal of Surgery 3 (1979): 685-691.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 12 (2015): 355-361.
- Inoue KO, Tobe T, Kan N, et al., Problems in the definition and treatment of early gastric cancer. Br J Surg 78 (1991): 818-821.
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surgical Laparoscopy & Endoscopy 4 (1994 ): 146-148.
- Katai H, Junki M, Hiroshi K, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 20 (2017): 699-708.
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 263 (2016): 28-35.
- Kim H-H, Han SU, Kim MC, et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncology 5 (2019): 506-513.
- Brar S, Law C, Robin M, et al. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. Journal of the American College of Surgeons 217 (2013): 347-357.
- Son T, Hyung WJ, Lee JH, et al. Minimally invasive surgery for serosa-positive gastric cancer (pT4a) in patients with preoperative diagnosis of cancer without serosal invasion. Surg Endosc 28 (2014): 866-74.
- Lee J, W Kim. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol 100 (2009): 693-698.
- Huscher CG, Mingoli A, Sgarzini G, et al. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg 194 (2007): 839-844.
- Brar S, Law C, Mcleod R, et al. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. J Am Coll Surg 217 (2013): 347-357.
- Jeong SH, KW Seo, JS Min. Intraoperative Tumor Localization of Early Gastric Cancers. J Gastric Cancer 21 (2021): 4-15.
- Tokuhara T, Nakata E, Tenjo T, et al. A novel option for preoperative endoscopic marking with India ink in totally laparoscopic distal gastrectomy for gastric cancer: A useful technique considering the morphological characteristics of the stomach. Mol Clin Oncol 6 (2017): 483-486.
- Matsui H, Ozkamoto Y, Nabeshima K, et al. Endoscopy-assisted gastric resection: a safe and reliable procedure for tumor clearance during laparoscopic high distal or proximal gastrectomy. Surg Endosc 23 (2009): 1146-1149.
- Hachisu T, Si Miyazaki, KiSe Hamaguchi. Endoscopic clip-marking of lesions using the newly developed HX-3L clip. Surg Endosc 3 (1989): 142-147.
- Kojima F, Sato T, Tsunoda S, et al. Development of a novel marking system for laparoscopic gastrectomy using endoclips with radio frequency identification tags: feasibility study in a canine model. Surg Endosc 28 (2014): 2752-2759.
- Kim HI, Hyung WJ, Lee CR, et al. Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for gastrectomy. Surg Endosc 25 (2011): 958-963.
- Tokuhara T, Nataka E, Tenjo T, et al. A novel option for preoperative endoscopic marking with India ink in totally laparoscopic distal gastrectomy for gastric cancer: a useful technique considering the morphological characteristics of the stomach. Molecular and clinical oncology 6 (2017): 483-486.
- Qi XD. Gastroscopic mucosal biopsy and carbon ink injection marking for determination of resection line on the gastric wall in stomach cancer. Chinese Journal of Oncology 11 (1989): 136-138.
- Yamazaki Y, Kanaji S, Takiguchi G, et al. Preoperative endoscopic tattooing using India ink to determine the resection margins during totally laparoscopic distal gastrectomy for gastric cancer. Surg Today 51 (2021): 111-117.
- Committee AT, Kethu SR, Banerjrr S, et al. Endoscopic tattooing. Gastrointest Endosc 72 (2010): 681-685.
- Wang SF, Cheng HT, Hsu JT, et al. Simple and Reliable Method for Gastric Subepithelial Tumor Localization Using Endoscopic Tattooing before Totally Laparoscopic Resection. Journal of Personalized Medicine 11 (2021).
- Atthaphorn T, Thawatchai A. Endoscopic tattooing of colorectal lesions: is it a risk-free procedure? World J Gastrointest Endosc 3 (2011): 256.
- Miyoshi N, Ohue M, Noura S, et al. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg Endosc 23 (2009): 347-351.
- Atthaphorn T, Thawatchai A. Endoscopic tattooing of colorectal lesions: is it a risk-free procedure? Am J Gastroenterol 3 (2011): 256.
- Mc Arthur CS, Roayaie S, Waye JD. Safety of preoperation endoscopic tattoo with india ink for identification of colonic lesions. Surg Endosc 13 (1999): 397-400.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 61 (2011): 69-90.
- Strong VE, Kyo YS, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 251 (2010): 640-646.
- Strong VE, Kyo YS, Park CH et al. Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol 107 (2013): 634-6340.
- Sasako M, Sano T, Yamaamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359 (2008): 453-462.
- Yang HK, YS Suh, HJ Lee. Minimally invasive approaches for gastric cancer-Korean experience. J Surg Oncol 107 (2013): 277-281.
- Strong VE, N Devaud, M Karpeh. The role of laparoscopy for gastric surgery in the West. Gastric Cancer 12 (2009): 127-131.
- Kumazu Y, Hayashi T, Yoshikawa T, et al. Risk factors analysis and stratification for microscopically positive resection margin in gastric cancer patients. BMC Surg 20 (2020): 95.
Article Views: 684
Journal Statistics





Discover More: Recent Articles
Grant Support Articles
© 2016-2024, Copyrights Fortune Journals. All Rights Reserved!