Varenicline Efficacy on Tobacco Dependence in Black/African Americans in the United States: A Systematic Review
Article Information
Charlene Mansour1,2, Victoria DeJaco, PharmD1,, Caitlyn Ahlberg, MD, MA8, Raiza Schreiber, RN1, Dina G. Lansey, MSN, RN3, Geeta Minocha, B.Sc.1,4,9, Shireen Khoury, MD, MPH1,7, Bekir Kaplan, MD6, Tugba Kaplan, MD10, Mandeep Jassal, MD5, Alejandra Ellison-Barnes, MD, MPH1,8, Panagis Galiatsatos, MD, MHS1,2,8*
1The Tobacco Treatment and Cancer Screening Clinic, the Johns Hopkins Health System, Baltimore, MD
2Division of Pulmonary and Critical Care Medicine, the Johns Hopkins School of Medicine, Baltimore, MD, USA
3Department of Oncology, the Johns Hopkins School of Medicine, Baltimore, MD, USA
4 Columbia Law School, New York, NY
5Division of Pulmonary, Department of Pediatrics, the Johns Hopkins School of Medicine, USA
6Institute for Global Tobacco Control, Department of Health, Behavior and Society, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA
7Division of Cardiology, the Johns Hopkins University School of Medicine, Baltimore, MD, USA
8Department of Medicine, the Johns Hopkins School of Medicine, Baltimore, MD, USA
9Wharton School, University of Pennsylvania, Philadelphia, PA, USA
10Anne Arundel Medical Center, Annapolis, MD, USA
*Corresponding author: Panagis Galiatsatos, The Tobacco Treatment and Cancer Screening Clinic, the Johns Hopkins Health System, Baltimore, USA.
Received: 11 April 2023; Accepted: 19 April 2023; Published: 16 May 2023
Citation: Charlene Mansour, Victoria DeJaco, PharmD, Caitlyn Ahlberg, MD, MA, Raiza Schreiber, RN, Dina G. Lansey, MSN, RN, Geeta Minocha, B.Sc, Shireen Khoury, MD, MPH, Bekir Kaplan, MD, Tugba Kaplan, MD, Mandeep Jassal, MD, Alejandra Ellison-Barnes, MD, MPH, Panagis Galiatsatos, MD, MHS. Varenicline Efficacy on Tobacco Dependence in Black/African Americans in the United States: A Systematic Review. Archives of Clinical and Biomedical Research. 7 (2023): 337-344.
View / Download Pdf Share at FacebookAbstract
Introduction: In 2018, the United States (US) achieved the lowest prevalence of active cigarette smoking since tracking began in 1965 at 13.8%. One factor that has influenced the decline in smoking is the advancement of pharmacotherapeutic treatments targeting the nicotine addiction component of tobacco dependence, specifically varenicline. However, it is unclear if the success of varenicline is evident in all diverse populations. We conducted a systematic review to evaluate the efficacy of varenicline based on race, specifically on Black/African Americans, with regards to smoking cessation.
Methods: In this systematic review, the online databases PubMed, Embase, Web of Science, and Scopus were searched from 2000 through July 20, 2021. We report varenicline’s efficacy on smoking cessation and tobacco dependence in Black/African American individuals.
Results: We identified three trials that recruited a majority of Black/ African American participants. In all three trials, varenicline was found to be effective and safe; however, abstinence rates varied by socioeconomic factors, genotyping, and adherence.
Conclusion: Moving forward, over-representation in clinical trials should be a priority for marginalized patients, who are disproportionately impacted by the associated adverse outcomes of tobacco dependence. Understanding barriers to clinical implementation of evidence-based pharmacotherapy should also be prioritized.
Keywords
Varenicline; Tobacco
Article Details
Introduction
The advancement of research regarding the neurobiological impact of chronic exposure to chemically enhanced nicotine from tobacco products has led to the development of novel pharmacological therapeutics as well as improvement of existing medication regimens [1-5]. At the time when the US Surgeon General issued the report on smoking cessation in 1990, the only medication that was FDA-approved for the indication of smoking cessation was the gum formulation of nicotine replacement therapy (NRT) [6]. Since that time, additional medications for tobacco dependence management including transdermal patches, lozenges, and nasal spray formulations of NRT have been approved along with the non-NRT medications of bupropion and varenicline [1,3,7]. While all of these pharmacotherapies have well-documented efficacy in assisting patients with their tobacco dependence, varenicline is more effective compared to transdermal NRT alone or to bupropion [8,9]. In 2018, the US achieved the lowest prevalence of active cigarette smoking since tracking began in 1965 at 13.8% [10]. Further, the decline in the prevalence of active cigarette smoking among adults is mirrored across racial categories—smoking among those of White race decreased from 42.2% in 1965 to 15.2% by 2017, while for Blacks, the rate declined from 46.0% to 14.9% over the same time frame [10]. While the relative declines in cigarette smoking appear to parallel one another in the aforementioned racial categories, there is a disparity in smoking-related disease outcomes; for instance, lung cancer disproportionally causes poorer outcomes in Black patients compared to White patients [11-14]. Further, recent epidemiological studies find that Black patients have lower quit rates than their White counterparts despite more quit attempts [15]. This will likely amplify current and near-future smoking-related health disparities [10, 16]. Therefore, it is imperative to evaluate varenicline as the leading pharmacotherapy agent for tobacco dependence management in the population that is being impacted more severely by cigarette consumption.
In this study, we performed a systematic review of varenicline and its efficacy on smoking cessation specifically in Black/African American persons in the United States (US). In regards to minority races in the US, Black/African American persons constitute the largest population and carry dire health outcomes related to smoking and tobacco dependence; therefore, evaluating the impact of varenicline on such a population may result in further insight on health equity pharmacotherapy management and approaches [10]. Studies were evaluated for the inclusion of Black/African American participants, consideration of sociodemographic factors, and efficacy of varenicline.
Methods
Data Sources
We searched for English-language articles published from 2000 through July 20, 2021, in four databases: PubMed, Embase, Web of Science, and Scopus. As varenicline was approved by the FDA in 2006 [9], we opted to start with the year 2000 to ensure capture of earlier trials of varenicline in the US.
Search methods to identify studies
Given the abundance of research studies on the efficacy of varenicline, we implemented a specific search strategy based on the PICOS criteria (Supplement) to identify studies that examined outcomes with the consideration of race. Two concepts guided the final search for pertinent studies using a combination of associated terms: 1) varenicline and 2) Blacks/African Americans. Each component part of the search strategy was developed using an amalgam of Medical Subject Headings (MeSH) and keyword terms in PubMed, then adapted for use in the other databases. Finally, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was employed during the conception of the systematic review and assisted in guiding articles reviewed and selected [17].
Eligibility Criteria
We sought to identify published work that documented the use of varenicline to increase smoking cessation among Black/African Americans. We included trials with the following characteristics: (1) Country: Studies conducted in the US. (2) Language: English. (3) Publication date: between 2000-July 2021. (4) Patient population: adult (18 years of age and older) smokers who self-identify as non-Hispanic/Latino and African American/Black in the US. (5) Intervention: the use of varenicline to aid in smoking cessation. Studies that used supplemental counseling in conjunction with varenicline were included. (6) Outcomes: primary and secondary outcomes related to smoking cessation (e.g., abstinence, quit attempts, quit rates, and number of cigarettes smoked, using both validated and self-reported measures). (7) Study design: studies were limited to primary research; however, study design was not restricted and could include randomized control trials, retrospective, prospective, cross-sectional, or longitudinal designs. (8) Peer-reviewed studies; abstracts-only were not included.
PICOS Framework
We employed the PICOS (Population, Intervention, Comparison, Outcome, Study Design) approach to determine the structure and scope of this systematic review. The characteristics of the PICOS approach are detailed in the eligibility criteria. The use of treatment comparisons was not a focus of this review; however, some studies used control/comparator groups to evaluate outcomes between Black/African American and White populations. Studies in which the efficacy of varenicline was not specifically investigated and/or varenicline was used in conjunction with interventions (other than counseling) were excluded. We only considered studies conducted within the US and in English to limit potential bias associated with the definition of race and if race is considered a proxy for other socioeconomic variables in studies outside of the US.
Data extraction, management, and analysis
CM independently extracted study characteristics from included studies. Data were extracted from these studies using a tailored standardized form to document study and intervention characteristics and results which included data on authors, publication year, primary outcomes, databases, number of patients, number of Black/African American patients, study years, total years, results, and methods. PG was involved in reviewing any studies in question. A range of qualitative and quantitative data was summarized descriptively and then synthesized by theme. VD aided with data management and analysis. A narrative synthesis has been provided to summarize and explain findings. For study quality, we utilized the GRADE approach for the systematic review [18].
Results
Study Selection
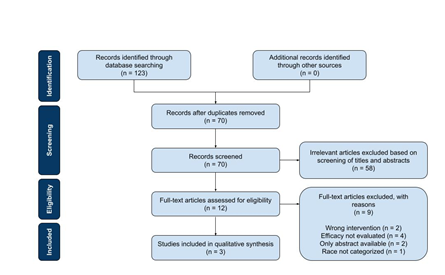
The search identified 123 studies of possible relevance and imported into RefWorks. Fifty-three duplicate articles were excluded by RefWorks. After screening studies on the basis of their titles and abstracts, 12 studies remained eligible for full-text assessment. Each article was then thoroughly reviewed for its focus on the efficacy of varenicline, inclusion of Black/African American participants, and analysis of race in outcomes. Of the 12 articles assessed, only 3 studies were consistent with the predefined selection criteria as above and included in the review (Figure 1).
Study Populations
A total of 1450 participants were included in this review, of which 635 were Black/African American. Table 1 summarizes the identified studies. Nollen et al. included 224 Black/African American participants, which comprised 50.0% of the total patients included in the study [19]. Chen et al. had 270 Black/African American participants, which comprised 32.9% of the total patients included in the study [20]. Ashare et al. had 141 Black/African American patients, which comprised 81.5% of the total patients included in the study [21]. Note that Ashare et al. had a predominantly Black/African American population in their study and did not categorize any other race. Of the three, two of the aforementioned studies disclosed how race was captured (self-reported) [19, 21].
|
Author (Year) |
Study Design |
Participants |
Blacks/AA Participants (%) |
Primary Outcome |
Secondary Outcome(s) |
|
Nollen et al. (2019) [19] |
Prospective intervention trial |
449 |
224 (50%) |
Self-reported 7-day point prevalence smoking abstinence, defined as no cigarettes for the previous 7 days. Biochemically verified by salivary cotinine. |
Salivary cotinine-verified 7-day point prevalence abstinence at weeks 4 and 12. |
|
Chen et al. (2020) [20] |
Placebo-controlled randomized clinical trial |
882 |
270 (32.9%) |
Self-reported 7-day point prevalence smoking abstinence, defined as no smoking (not even a puff) for at least 7 days before the assessment. Biochemically verified by exhaled CO. |
A 7-day point prevalence abstinence with CO verification at 6 months, 7-day point prevalence abstinence at 1 year by self-report, adverse effects, and adherence. |
|
Ashare et al. (2019) [21] |
Placebo-controlled randomized clinical trial |
179 |
141 (81.5%) |
Self-reported 7-day point prevalence smoking abstinence, defined as no tobacco use during the 7 days preceding the assessment. Biochemically verified by breath CO. |
A 7-day point-prevalence abstinence at week 18, continuous abstinence rates (with CO) from Weeks 9-12, 9-18, and 9-24 (defined as no self-reported tobacco use for the duration of the timeframe), and time to relapse across the 24-week trial. |
|
AA = African American. CO = carbon monoxide. |
|||||
Table 1: Characteristics of studies included in systematic review.
Study Designs
Nollen et al. conducted a prospective intervention trial [19] with evidence cited regarding varenicline’s effectiveness for smoking cessation among White participants. Nollen et al. assigned patients to varenicline treatment only and without a pharmacotherapy control arm. Rather, the authors sought to investigate differences in efficacy between White participants and Black participants.
Chen et al. and Ashare et al. both conducted placebo-controlled randomized clinical trials [20, 21]. Chen et al. randomized patients to one of three treatments in a 1:1:1 fashion: (i) varenicline, (ii) nicotine patches and nicotine lozenges, or (iii) placebo varenicline or placebo nicotine patches/lozenges [20]. Ashare et al. randomized patients (1:1) to varenicline or placebo [21]. In all three studies, participants received behavioral counseling sessions in conjunction with pharmacotherapy. The primary outcome of all studies was the 7-day point prevalence smoking abstinence, both self-reported and biochemically verified. Nollen et al. biochemically verified abstinence by salivary cotinine levels <15 ng/mL [19]. Chen et al. and Ashare et al. both verified abstinence by exhaled carbon monoxide (CO) levels less than 8 ppm [20,21]. In regards to handling missing outcome data, either loss to follow-up or lack of objective data provided (e.g. CO measurements), all three studies considered such participants as ongoing active smokers.
Study Description and Outcomes
Nollen et al.19
This prospective intervention trial stratified participants on self-reported race followed by further separation within race based on age (<40, ≥40 years) and sex, resulting in eight categories (Black males <40 years, Black males ≥40 years, Black females <40 years, Black females ≥40 years, White males <40 years, White males ≥40 years, White females <40 years, White females ≥40 years). The intervention included a total of 12 weeks of varenicline, six concurrent smoking cessation counseling sessions through week 16, and follow-up through week 26. Inclusion criteria were those individuals who smoked 3-20 cigarettes per day and were interested in quitting smoking. Black participants smoked an average of 12.5 cigarettes per day compared to White participants who smoked an average of 16.9 cigarettes per day. Results demonstrated a statistically significant primary outcome, with Black participants less likely to achieve abstinence than White participants at week 26 (14.3% of Blacks and 24.4% of Whites, p = 0.007).
In terms of secondary outcomes, the inclusion of socioeconomic factors, treatment process, and smoking characteristics in multi-variable adjusted models attenuated the association of race with abstinence. Specifically, decreased abstinence rates were associated with socioeconomic factors including lower income, lack of home ownership, and greater neighborhood issues and conflicts, which were more prevalent among Black participants. With such a finding, the authors emphasized that the influence of non-biological social factors (e.g. socioeconomic, treatment process, and smoking characteristics) may play a more a significant role than race alone. In terms of side effects, Black and White participants reported a similar prevalence of moderate to severe side effects that could be attributed to varenicline; interestingly, abnormal dreams were experienced more frequently by Black participants.
Chen et al.20
This was a prospective stratified randomization trial that was genotype based. Each participant underwent genetic analysis via blood sample. Of the 822 participants enrolled in the trial, 454 had GG genotypes and 368 had GA/AA genotypes; 516 smokers were of European ancestry (EA) and 306 of non-EA, composed of 270 smokers of African American ancestry and the final 36 smokers reported other ancestry. Participants were randomly assigned based on CRNA5 rs16969968 genotypes (GG vs. GA/AA) to one of three treatments for 12 weeks in a 1:1:1 ratio: (i) varenicline tartrate, (ii) nicotine patches with nicotine lozenges, or (iii) placebo varenicline tartrate or placebo nicotine patches/lozenges, resulting in six cohorts. Cessation counseling occurred for all groups and follow-up assessments occurred for up to 12 months after the scheduled quit date., Income was recorded in addition to race, with 50.2% of the participants reporting an annual income ≥ $30,000/y. Eligibility for the study included ages 21 years and older, at least 5 cigarettes smoked per day, and were actively seeking treatment for smoking cessation. The average cigarettes smoked per day was 17.6. The 7-day point prevalence of abstinence found both cNRT and varenicline were statistically significant when compared to placebo, while age (p = 0.12) and sex (p = 0.83) were not statistically significant the rate of abstinence. Race significantly predicted abstinence, where abstinence rates were lower within the non-EA smokers cohort than EA smokers (OR = 0.65, 95% CI, 0.44-0.96, p = 0.030). Both cNRT and varenicline were effective compared with placebo within each racial group. In terms of the interaction of genotype and pharmacotherapy on smoking cessation outcomes, both cNRT and varenicline vs. placebo were efficacious regardless of rs16969968 genotypes (GG vs. GA/AA) in smokers of EA indicating no genotype-by-treatment interaction for the primary outcome of abstinence (p = 0.91). The lack of genotype-by-treatment interaction was confirmed after adjustments for age and sex within the EA cohort. In Black/African American smokers, genotype-by-treatment interaction was statistically significant for the primary outcome of abstinence (p = 0.0049). Within smokers that had a rs16969968 GG genotype, cNRT versus. placebo was efficacious (p = 0.029), but varenicline vs. placebo was not significant (p = 0.24). The comparison of paired cNRT vs. varenicline did not demonstrate significance (p = 0.27). In contrast, for White participants with the alternate genotype of rs16969968 GA/AA, efficacy of varenicline vs. placebo was demonstrated, but not when comparing cNRT vs. placebo. Unlike the Black/African American group, comparison of the pairwise varenicline vs. cNRT was significant (p = 0.0052). After adjusting for age and sex, similar results of the genotype-by-treatment interaction were confirmed (p = 0.0035). Regarding adverse events, there was no significant difference in overall adverse effect severity score in Black/African American smokers across medication groups versus placebo groups, paired cNRT vs. varenicline, or separate genotype groups (GG vs. GA/AA). Also, no significant genotype-by-treatment interaction was noted. There were similar findings for EA participants.
Ashare et al. 21
Ashare et al. conducted a single-site phase 3 clinical trial with a randomized, double-blind, and placebo-controlled structure, of people living with HIV (PLWH) on antiretroviral therapy (ART) who were daily smokers seeking treatment. Participants were randomized to varenicline or placebo for 12 weeks and all participants were offered six concurrent smoking cessation behavioral counseling sessions. Seven-day point prevalence abstinence (confirmed with breath CO) at weeks 12 and 24 acted as the primary outcome. Secondary outcomes included continuous abstinence and time to relapse. Treatment-related side effects, blood pressure, adverse events, ART adherence, and viral load were followed as safety measures. In addition to participant HIV and ART status, participants were required to be >18 years of age and smoke a minimum of 5 cigarettes per day. Of note, the average number of cigarettes smoked by the participants was 11.5 cigarettes per day. The authors recruited 179 participants, with 141 (81.5%) identifying as Black/African American. In terms of primary outcome achievement, varenicline participants reported significantly higher abstinence rates at Week 12 (p = 0.001) when compared to placebo. However, this effect was lost by Week 24 (p = 0.20). No differences on safety measures were noted between varenicline and placebo, although medication adherence within the study was greater in the placebo arm (138.0 + 40.3 pills) with 75.6% of participants reported to have taken >80% of placebo pills, compared to the varenicline arm (124.0 + 51.5) with only 58.4% of participants taking varenicline consistently (p = 0.02).
Discussion
With varenicline identified as the leading pharmacotherapy for tobacco dependence management9, its efficacy across diverse populations must be evaluated to reaffirm confidence in the medication as a potent, evidence-based treatment. Within such diverse populations, the sociodemographic variable of race should be considered. Race is used as a proxy in the US for more complex social, demographic, and economic factors, all of which may influence smoking cessation. A deeper understanding of this factor’s effect on varenicline’s efficacy may better equip clinicians to recognize barriers attenuating the success of varenicline for Black/African American patients. Our systematic review of varenicline and its efficacy for smoking cessation in Black/African American persons found that varenicline achieved statistically significant cessation. Therefore, a clinical understanding of the implication of these findings is warranted. While smoking prevalence appears to have decreased in a similar fashion between racial populations in the US, smoking-related health issues have disproportionately affected Black/African American patients. These patients suffer from one of the highest incidence and mortality rates of lung cancer among all ethnic and racial groups; notably, lung cancer is the leading cause of death among Black/African American men [22]. Studies suggest that Black/African American individuals in the US with chronic obstructive pulmonary disease (COPD) have worse pulmonary functional status, more significant lung function impairment, and worse COPD-related exacerbations as compared to non-Hispanic White persons [23-25]. Therefore, in order to achieve equal and equitable outcomes compared to other racial categories, the urgent need to manage tobacco dependence in Black/African Americans warrants the use of evidence based interventions (e.g. varenicline pharmacotherapy). With this systematic review, we reaffirm confidence in the clinical utility of varenicline for such patients. Cost is often cited as a barrier to the successful implementation and wide-spread dissemination of varenicline in the US.9 This was highlighted in the most recent tobacco dependence guidelines, where accessibility (e.g. over-the-counter or need for prescription) and lower costs were seen as important factors to consider for a pharmacotherapy in addition to efficacy and safety [9]. In the US, attempts to mitigate the often-prohibitive cost of varenicline vary by state and insurance status; cost to the patient is impacted by insured status as well as of the quality and extent of coverage, which may not fully cover varenicline. Medication cost is important to consider when interpreting clinical trials on varenicline, as affordability may disproportionately impact those uninsured, underinsured, and limited by the prohibitive cost of medications [26, 27].
Mental health morbidities are more severe, persistent, and disabling among Black/African American individuals [28, 29]. Those suffering from mental health disorders have higher rates of smoking as compared to those without mental health disorders [30].Therefore, a concern found within all three trials discussed is the exclusion of patients with advanced mental health issues, especially given that the trials were conducted post-removal of the black box warning on varenicline’s potential for neuropsychiatric events (i.e. depression and suicide) in 2016 [31]. Future studies should seek to assess the safety and efficacy of varenicline in Black/African American smokers with significant mental health diseases. There are limitations to note with our systematic review. First, we chose to analyze only race, specifically towards the largest minority race in the US that has significant smoking-related health outcomes. Future studies should take into account other sociodemographic factors and their respective populations, such as ethnicity and neighborhood socioeconomic status [32,33]. Second, it will be vital to evaluate the efficacy of varenicline beyond 6 months in Black/African American patients, as current patients who smoke may need more time for cessation [9]. As the 6-month margin is an arbitrary end-point for clinical management, as reflected with the most recent guideline, having clinical research reflect clinical practice will assure more precise intervention with varenicline for patients based on sociodemographic factors and clinical severity of tobacco dependence. Third, generalizing studies on race globally will always carry limitations given the notion that race carries different meanings and definitions across nations [34-36]. However, we believe the general concepts of our study can be generalized for the purpose of exploring varenicline’s impact on specific social groups globally, even if the findings themselves cannot be extrapolated.
Conclusion
In this systematic review of varenicline’s efficacy and safety for Black/African American patients, we found that the pharmacotherapy is a successful evidence-based option in tobacco dependence management. This finding supports the use of varenicline as an effective therapeutic option for Black/African American patients who smoke. Future studies should further address knowledge gaps in the care of Black/African American patients, such as varenicline safety and efficacy for those with co-morbid significant mental health issues. Further evaluation of varenicline’s impact on specific smoking phenotypes and subpopulations will improve the equitable and successful implementation of this therapy as part of an overall smoking-cessation strategy in the US.
Authors’ Contributions
PG, VD, CM underwent the initial systematic review. CM reviewed all articles, with VD weighing in on relevant manuscripts. PG assisted in reviewing any additional manuscripts. PG, VD, CM contributed to the writing of the manuscript. All authors contributed in the concept of the manuscript along with reviewing and editing. DGL and AEB reviewed and edited the final version of the manuscript.
References
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49 (2009): 57-71.
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med 121 (2008): S3-10.
- Cahill K, Lindson-Hawley N, Thomas KH, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev (2016): CD006103.
- Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391(1998):173-177.
- Polosa R, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci 32 (2011): 281-289.
- The Surgeon General's 1990 Report on The Health Benefits of Smoking Cessation. Executive Summary. MMWR Recomm Rep 39 (1990): 1-12.
- Leas EC, Pierce JP, Benmarhnia T, et al. Effectiveness of Pharmaceutical Smoking Cessation Aids in a Nationally Representative Cohort of American Smokers. J Natl Cancer Inst 110 (2018): 581-587.
- Galiatsatos P, Garfield J, Melzer AC, et al. Summary for Clinicians: An ATS Clinical Practice Guideline for Initiating Pharmacologic Treatment in Tobacco-Dependent Adults. Ann Am Thorac Soc 18 (2021): 187-190.
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating Pharmacologic Treatment in Tobacco-Dependent Adults. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 202 (2020): e5-e31.
- In: Smoking Cessation: A Report of the Surgeon General. Washington (DC) (2020).
- Jemal A, Miller KD, Sauer AG, et al. Changes in Black-White Difference in Lung Cancer Incidence among Young Adults. JNCI Cancer Spectr 4 (2020): 055.
- Lake M, Shusted CS, Juon HS, et al. Black patients referred to a lung cancer screening program experience lower rates of screening and longer time to follow-up. BMC Cancer 20 (2020): 561.
- Pasquinelli MM, Tammemagi MC, Kovitz KL, et al. Risk Prediction Model Versus United States Preventive Services Task Force Lung Cancer Screening Eligibility Criteria: Reducing Race Disparities. J Thorac Oncol 15 (2020): 1738-1747.
- Sin MK. Lung cancer disparities and African-Americans. Public Health Nurs 34 (2017): 359-362.
- Babb S, Malarcher A, Schauer G, et al. Quitting Smoking Among Adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep 65 (2017): 1457-1464.
- Warren GW, Alberg AJ, Kraft AS, et al. The 2014 Surgeon General's report: "The health consequences of smoking--50 years of progress": a paradigm shift in cancer care. Cancer 120 (2014): 1914-1916.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372 (2021): n71.
- Brozek JL, Canelo-Aybar C, Akl EA, et al. GRADE Guidelines 30: the GRADE approach to assessing the certainty of modeled evidence-An overview in the context of health decision-making. J Clin Epidemiol 129 (2021): 138-150.
- Nollen NL, Mayo MS, Sanderson Cox L, et al. Factors That Explain Differences in Abstinence Between Black and White Smokers: A Prospective Intervention Study. J Natl Cancer Inst 111 (2019): 1078-1087.
- Chen LS, Baker TB, Miller JP, et al. Genetic Variant in CHRNA5 and Response to Varenicline and Combination Nicotine Replacement in a Randomized Placebo-Controlled Trial. Clin Pharmacol Ther 108 (2020): 1315-1325.
- Ashare RL, Thompson M, Serrano K, et al. Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug Alcohol Depend 200 (2019): 26-33.
- American Cancer Society. Cancer facts & figures for African Americans 2022. (2022).
- Ejike CO, Woo H, Galiatsatos P, et al. Contribution of Individual and Neighborhood Factors to Racial Disparities in Respiratory Outcomes. Am J Respir Crit Care Med 203 (2021): 987-997.
- Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health 65 (2011): 26-34.
- Sarrazin MV, Cannon KT, Rosenthal GE, et al. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. J Natl Med Assoc 101 (2009): 656-662.
- Sohn H. Racial and Ethnic Disparities in Health Insurance Coverage: Dynamics of Gaining and Losing Coverage over the Life-Course. Popul Res Policy Rev 36 (2017): 181-201.
- Link CL, McKinlay JB. Only half the problem is being addressed: underinsurance is as big a problem as uninsurance. Int J Health Serv 40 (2010): 507-523.
- Williams DR, Gonzalez HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry 64 (2007): 305-315.
- Alang SM. Mental health care among blacks in America: Confronting racism and constructing solutions. Health Serv Res 54 (2019): 346-355.
- Prochaska JJ, Das S, Young-Wolff KC. Smoking, Mental Illness, and Public Health. Annu Rev Public Health 38 (2017): 165-185.
- Kelley KJ. FDA Removes Black Box Warning from Varenicline's Label. NEJM Journal Watch (2016).
- Galiatsatos P, Brigham E, Krasnoff R, et al. Association between neighborhood socioeconomic status, tobacco store density and smoking status in pregnant women in an urban area. Prev Med 136 (2020):106107.
- Galiatsatos P, Kineza C, Hwang S, et al. Neighbourhood characteristics and health outcomes: evaluating the association between socioeconomic status, tobacco store density and health outcomes in Baltimore City. Tob Control 27 (2018): e19-e24.
- Ford ME, Kelly PA. Conceptualizing and categorizing race and ethnicity in health services research. Health Serv Res 40 (2005): 1658-1675.
- Smedley A, Smedley BD. Race as biology is fiction, racism as a social problem is real: Anthropological and historical perspectives on the social construction of race. Am Psychol 60 (2005): 16-26.
- Witzig R. The medicalization of race: scientific legitimization of a flawed social construct. Ann Intern Med 125 (1996): 675-679.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 