Dermoscopic Features of Subcorneal Hematoma on the Palms and Soles: Differences from Acral Melanoma
Article Information
Sang-Hyeon Won1, Jeongwu Seong1, Jungsoo Lee3,4, Kihyuk Shin3,4, Hoon-Soo Kim1,2, Hyun-Chang Ko3,4, Byung-Soo Kim1,2,Moon-Bum Kim1,2*
1Department of Dermatology, School of Medicine, Pusan National University, Busan, Korea
2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
3Department of Dermatology, Pusan National University Yangsan Hospital, Yangsan, Korea
4Research Institute for Convergence of Biomedical Science and Technology, Yangsan, Korea
*Corresponding author: Moon-Bum Kim. Department of Dermatology, School of Medicine, Pusan National University, Busan, Korea
Received: 28 September 2023; Accepted: 03 October 2023; Published: 25 October 2023.
Citation: Sang-Hyeon Won, Jeongwu Seong, Jungsoo Lee, Kihyuk Shin, Hoon-Soo Kim, Hyun- Chang Ko, Byung-Soo Kim, Moon-Bum Kim. Dermoscopic Features of Subcorneal Hematoma on the Palms and Soles: Differences from Acral Melanoma. Archives of Clinical and Biomedical Research. 7 (2023): 537-542.
View / Download Pdf Share at FacebookAbstract
Purpose: The diagnosis of subcorneal hematoma (SH) can be challenging because the clinical presentation of SH can resemble melanocytic lesions. Few studies have examined the characteristic dermoscopic features of SH, but a more detailed large-scale study is needed to overcome the diagnostic challenge of differentiating it from acral melanoma.
Materials and Methods: We evaluated the clinical and dermoscopic features of 50 SH lesions from 43 patients at the Pusan National University Hospitals (Busan and Yangsan).
Results: In the color analysis, 86% of cases showed the bruise color sign; 7 cases had a single color (red to purple: 2; black: 1; brown: 4). Typical dermoscopic features of SH, acral nevi, and acral melanoma-associated patterns were observed in 60%, 0%, and 72% of lesions, respectively. Hematoma-associated patterns were homogenously red to black with or without satellite globules (32%) and pebbles on the ridges (28%). Acral melanoma-associated patterns showed a parallel ridge pattern (PRP) (52%), irregular dots and globules (50%), polychromia (34%), asymmetry (24%), irregular blotches (10%), and ulcers (10%). No case showed bluewhite veils, regression structures, atypical vascular patterns, or irregular fibrillar patterns. The bruise color sign was positive in most cases with acral melanoma-associated patterns (88.9%).
Conclusion: This study revealed the positive (bruise color sign, distinct PRP, and polychromia) and negative (blue-white veil, regression structures, atypical vascular pattern, and irregular fibrillar pattern) dermoscopic features of SH. Cases that are ambiguous between SH and acral melanoma can be distinguished based on these features.
Keywords
Bruise; Color; Dermoscopy; Hematoma; Melanoma
Article Details
1. Introduction
Palmoplantar subcorneal hematoma (SH) is a common pigmented lesion on acral volar skin, and may be caused by memorable trauma or repeated microtrauma [1]. SH patients commonly do not recall a distinct trauma to the affected region and are consequently anxious about skin cancer, especially acral melanoma [1, 2]. Under these circumstances, dermatologists can have a diagnostic dilemma because SH often clinically and dermoscopically resembles acral melanoma [3]. To solve this problem, previous studies have attempted to delineate the characteristic dermoscopic features of SH. Structureless, red-black, homogenous pigmentations with satellite globules at the periphery have been proposed as the most typical features. Acral nevus or melanoma-like features, various colors, and other characteristics have also been noted [1, 2]. However; these studies were neither systematic nor done on a large scale.
2. Materials and methods:
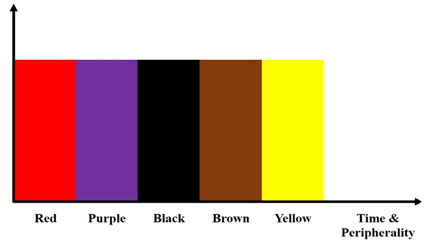
Clinical and dermoscopic SH data were collected in two tertiary university hospitals (Pusan National University Hospital [Busan and Yangsan]) for approximately 10 years (2011–2020). The diagnosis of SH was confirmed by a scratch test, histopathologic examination, or observing its fading at a subsequent follow-up. Dermoscopic images were acquired at 10-fold magnification with a dermoscope (DermLite DL3N; 3Gen Inc., San Juan Capistrano, CA, USA) attached to a digital camera (Sony Cybershot DSC- DSC-W810, 6x optical zoom, 20.1 megapixels; Sony Corporation, Tokyo, Japan). The assessed clinical data included age, sex, number of lesions, location, trauma history, and clinical diagnosis. The dermoscopic images were assessed by two dermoscopists (S-H Won and M-B Kim), focusing on its colors and dermoscopic patterns. For the SH colors, we coined the term “bruise color sign.” The idea of this sign was derived from the time-dependent color changes of a bruise on nonvolar skin. Unlike the usual bruise colors (pink and red, blue and dark purple, pale green, yellow and brown), SH showed black but no blue/green colors [4]. SH colors can vary according to time and to the periphery of SH lesions (Figure. 1).
The bruise color sign was considered to be positive when we found more than two of the four SH colors (red to purple, black, brown, and yellow colors). Dermoscopic patterns were divided into three categories: hematoma-associated, acral nevus-associated and acral melanoma-associated patterns. Hematoma-associated patterns were homogenous (red to black) with or without satellite globules and pebbles on the ridges [1, 2, 5]. Acral nevus-associated patterns were parallel-furrow, fibrillar, lattice, homogeneous (light brown), globular (not associated with a parallel pattern), and crista-dotted patterns [6]. Acral melanoma-associated patterns were parallel ridge pattern (PRP), irregular dots and globules, polychromia (≥ 4 colors), asymmetry, irregular blotches, ulcers, blue-white veil, regression structures, atypical vascular pattern, and irregular fibrillar pattern [7]. All analyses were performed using SPSS (version 25.0, IBM Corp., Armonk, NY, USA). Pearson’s chi-squared test was performed to evaluate the difference in dermoscopic patterns according to the presence or absence of trauma history and hand (non-weight bearing) or foot (weight-bearing) location. In all analyses, P<0.05 was considered statistically significant. The study was approved by the institutional review board (IRB No. 2102-018-100).
3. Results
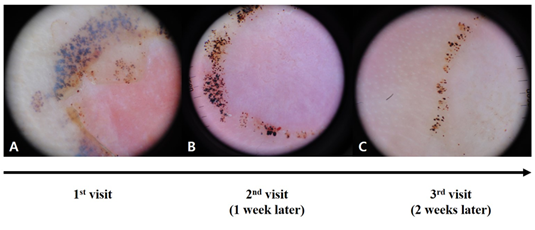
Fifty SH lesions from 43 patients were analyzed in this study (Table 1). A single SH was found in 38 patients (88.4%), and multiple SHs were observed in five patients (11.6%). The mean age was 42.1±22 (range, 3–76) years, and the male to female ratio was 1.39 (25:18). The most common location was the sole (n=20, 40%), followed by the toe (n=11, 22%), heel (n=10, 20%), finger (n=8, 16%), and palm (n=1, 2%). There was no significant difference in the dermoscopic patterns between palmar and plantar lesions. Most patients could not remember any trauma event, while 37.2% (16/43) of the patients provided the following trauma histories: soccer (7/16, 43.8%), jogging (3/16, 18.8%), stabbing incident (2/16, 12.5%), car accident (1/16, 6.3%), foot massage (1/16, 6.3%), improper footwear (1/16, 6.3%), and farming (1/16, 6.3%). There was no significant association between the presence or absence of trauma history and dermoscopic patterns of SH. As for the clinical diagnosis, most cases were confirmed as SH, but there were a few incidences of acral nevi (n=7, 14%) and acral melanoma (n=5, 10%). To confirm the diagnosis of SH, a scratch test was performed in two cases, and a skin biopsy was performed in 16 cases. The remaining 32 cases were followed-up at 1-week intervals until weeks 4-6 to confirm the diagnosis of SH (Figure. 2). As time went by, SH showed changing colors from red-purple to black-brown to yellow. The dermoscopic findings of SH are summarized in Tables 2 and 3. In the color analysis, most cases showed the bruise color sign (43/50, 86%), except for seven cases with a single color (red to purple: 2; black: 1; brown: 4). the colors of the bruise color sign were composed of two (21/43, 48.9%), three (5/43, 11.6%), or four colors (17/43, 39.5%). In 21 cases with two colors, red and black were most commonly found (n=12), followed by brown and yellow (n=6), black and brown (n=2), and red and yellow (n=1) colors. All five cases with three colors included red. To sum up, a red hue was found in 37 cases (37/50, 74%). Three-fifths of cases (30/50, 60%) showed hematoma-associated dermoscopic patterns with a red to black homogenous pattern with or without satellite globules (16/50, 32%) and pebbles on the ridges (14/50, 28%). Satellite globules were found in 15 cases (15/50, 30%) composed of pebbles on the ridges (n=9), red to black homogenous pattern (n=4), and PRP without pebbles (n=2). Of 16 cases with a red to black homogenous pattern, only four cases showed satellite globules. As for acral nevus-associated dermoscopic patterns, no case showed parallel-furrow, fibrillar, lattice, homogeneous (light brown), globular (not associated with a parallel pattern), or crista-dotted patterns Thirty-six cases (36/50, 72%) showed more than one pattern of the acral melanoma-associated dermoscopic patterns; these were PRP (26/50, 52%), irregular dots and globules (25/50, 50%), polychromia (17/50, 34%), asymmetry (12/50, 24%), irregular blotches (5/50, 10%), and ulcers (5/50, 10%). No case showed a blue-white veil, regression structures, an atypical vascular pattern, or an irregular fibrillar pattern (Table 3 and Figure. 3). Of the 26 cases with a PRP, 14 had pebbles on the ridges. The other 12 showed various colors, including red (n=3), black (n=3), and brown (n=6). Regarding brown PRP, four cases had PRP with a brick wall-like structure (Figure 3). Seven cases with no bruise color sign had a brown to black homogenous pattern (n=3), brown PRP with a brick wall-like structure (n=2), red PRP (n=1), and red irregular dots and globules (n=1).
Table 1: Clinical data of the study population with subcorneal hematoma
|
Contents |
n (%) or variables |
|
Patients |
43 |
|
SH lesions |
50 |
|
Single |
38 (88.4) |
|
Multiple |
5 (11.6) |
|
Age (years), mean±SD |
42.1±22 |
|
Male to female ratio |
1.39 (25:18) |
|
Location of SH lesions |
|
|
Sole |
20 (40) |
|
Toe |
11 (22) |
|
Heel |
10 (20) |
|
Finger |
8 (16) |
|
Palm |
1 (2) |
|
Trauma history |
|
|
Memorable |
16 (37.2) |
|
Non-memorable |
27 (62.8) |
|
Clinical diagnosis |
|
|
Subcorneal hematoma |
38 (76) |
|
Acral melanocytic nevi |
7 (14) |
|
Acral melanoma |
5 (10) |
SD, standard deviation; SH, subcorneal hematoma
Table 2: Colors of subcorneal hematoma
|
Colors |
n (%) |
|
Single |
7 (14) |
|
Red to purple |
2 |
|
Black |
1 |
|
Brown |
4 |
|
Bruise color sign |
43 (86) |
|
Two colors |
21 |
|
Red and black |
12 |
|
Brown and yellow |
6 |
|
Black and brown |
2 |
|
Red and yellow |
1 |
|
Three colors |
5 |
|
Four colors |
17 |
Table 3: Dermoscopic patterns of subcorneal hematoma
|
Dermoscopic patterns |
n (%) |
|
Hematoma-associated |
30 (60) |
|
Red to black homogenous pattern |
16 (32) |
|
with satellite globule |
4 |
|
without satellite globule |
12 |
|
Pebbles on the ridges |
14 (28) |
|
with satellite globule |
9 |
|
without satellite globule |
5 |
|
Acral nevus-associated |
0 (0) |
|
Acral melanoma-associated |
36 (72) |
|
PRP |
26 (52) |
|
Irregular dots and globules |
25 (50) |
|
Polychromia |
17 (34) |
|
Asymmetry |
12 (24) |
|
Irregular blotches |
5 (10) |
|
Ulcer |
5 (10) |
PRP, parallel ridge pattern
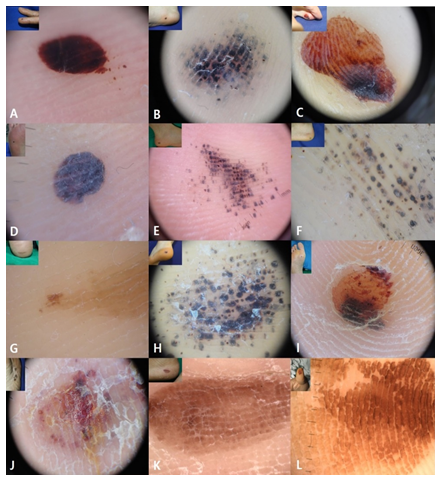
Figure 3: Colors and dermoscopic patterns of subcorneal hematoma. A, Bruise color sign (2 colors; red and black) and red to black homogenous pattern with satellite globule. B, Bruise color sign (3 colors; red-purple, black, and brown) and pebbles on the red ridges without satellite globule. C, Bruise color sign (4 colors; red, black, brown, and yellow), red PRP, and asymmetry. D, Red to black homogenous pattern without satellite globule. E, Pebbles on the ridges with satellite globules. F, Pebbles on the yellow ridges without satellite globules and polychromia. G, Brown to yellow PRP. H, Irregular dots and globules. I, Irregular blotches. J, Ulcer. K, Brown PRP with a brick wall-like structure. L, Brown PRP with a brick wall-like structure.
Discussion:
Palmoplantar SH is a well-known mimicker of acral melanoma [1]. When a pigmented skin lesion is found on the palms or soles, most Asian patients, including Koreans, seem to be very anxious due to the possible risk of acral melanoma [7]. This is because the acral volar skin is the most common site of malignant melanoma in non-white populations [5]. Therefore, a few studies have attempted to delineate the characteristic dermoscopic findings of SH [1, 2]. Although these studies have revealed some characteristic dermoscopic findings of SH, more specific and systematic studies are needed to delineate the exact ratios and differences of the typical dermoscopic features of SH and acral nevi or melanoma-associated patterns. Dermoscopy is a helpful, non-invasive tool that improves diagnostic accuracy in pigmented skin lesions [8-10]. Although SH is a common pigmented lesion on acral volar skin, studies on the dermoscopic features of SH are rare. Saida et al [5]. Were the first to describe the dermoscopic features of SH and coined the term “pebbles on the ridges,” denoting reddish-black droplets distributed on the ridges of skin markings. Since then, only two related original studies have been reported [1, 2]. Zalaudek et al [1]. Described the dermoscopic features of 15 SH lesions. They revealed that the most common color of SH was red-black (40%), followed by brown-black (13.3%), and gray-black (13.3%). In their study, the most common dermoscopic patterns were the homogenous pattern (8/15, 53.3%), followed by the globular pattern (7/15, 46.7%), the PRP (6/15, 40%), the parallel furrow (1/15, 6.7%) and the fibrillar pattern (1/15, 6.7%). Elmas et al.2 described the dermoscopic features of 20 SH lesions and reported that the most common color was red-black (45%), followed by brown (20%) and red (15%). They revealed that the most common dermoscopic pattern was the homogenous pattern (13/20, 65%), followed by the globular pattern (11/20, 55%), and the PRP (8/20, 40%). No patients showed parallel furrow or fibrillar patterns. However, these studies were performed on a small scale and provided insufficient results for the differentiation from acral melanoma [1, 2]. In this study, we coined the term bruise color sign after observing similar colors to bruises or contusions on nonvolar skin. Compared to typical bruise colors (pink and red, blue and dark purple, pale green, yellow and brown), SH showed black but no blue/green colors [4]. We speculated that the reason for this was the “Tyndall effect” considering that the depth of SH is much shallower than that of a usual bruises or contusions. Over four-fifths (43/50, 86.0%) of patients in this study showed the bruise color sign, of which the red hue was the most common (35/43, 81.4%). In addition, the bruise color sign was positive in most cases with acral melanoma-associated dermoscopic patterns (32/36, 88.9%) (Table 4).
Table 4: The correlation between acral melanoma-associated patterns and bruise color sign
|
Acral melanoma-associated (n=36) |
||||||||
|
PRP (n=26) |
Irregular dots and globules (n=25) |
Polychromia (n=17) |
Asymmetry (n=12) |
Irregular blotches (n=5) |
Ulcer (n=5) |
|
||
|
Bruise color sign |
+ |
23 |
24 |
17 |
12 |
5 |
5 |
|
|
- |
3 |
1 |
0 |
0 |
0 |
0 |
||
PRP, parallel ridge pattern
This provides a useful tool for SH that shows acral melanoma-associated dermoscopic patterns. In this study, approximately three-fourths (37/50, 74.0%) of patients showed a red hue, which was also predominant (35/43, 81.4%) in the bruise color sign. A red hue is not common in acral melanoma except in irregular polymorphic or hairpin-like vessels, ulcers, or milky red areas; a red hue, especially as part of the bruise color sign, can also be useful for the differential diagnosis with acral melanoma [7, 11]. Different from previous studies, this study clearly divided the dermoscopic patterns of SH into three categories: hematoma-associated, acral nevus-associated, and acral melanoma-associated patterns. In this study, we adopted only two typical hematoma-associated patterns (red to black homogenous pattern with or without satellite globules and pebbles on the ridges). Three-fifths of cases (30/50, 60%) showed one of these hematoma-associated dermoscopic patterns. Satellite globules, red to black globules at the periphery, can be an important dermoscopic pattern for SH. We found this pattern in 15 cases (15/50, 30%), which consisted of pebbles on the ridges (n=9), a red to black homogenous pattern (n=4), and PRP without pebbles (n=2). Compared to the study by Zalaudek et al [1] this ratio was somewhat lower (hematoma-associated dermoscopic patterns: 60% vs. 80%, satellite globules: 30% vs. 46.7%). Although approximately half of the cases had a globular pattern in other studies (Zalaudek et al [1]: 46.7%, Elmas et al.2: 55%), and quite a few cases showed parallel furrow and fibrillar patterns (Zalaudek et al [1]: 13.3%), we could not find any case with acral nevus-associated dermoscopic patterns. Although we found 25 cases with multiple dots and globules, they did not correspond to the globular pattern of acral nevomelanocytic nevus that is not typically associated with a parallel pattern. In these cases, the patterns were irregular dots and globules, such as in the melanoma pattern but not in acral nevus. Some of them (n=14) were associated with PRP. In this study, 36 cases (36/50, 72%) showed acral melanoma-associated dermoscopic patterns. Of these, PRP (26/50, 52%) was the most common, followed by irregular dots and globules (25/50, 50%), polychromia (17/50, 34%), asymmetry (12/50, 24%), irregular blotches (5/50, 10%), and ulcers (5/50, 10%). We could not find any case with blue-white veils, regression structures, atypical vascular pattern, or irregular fibrillar patterns. Mun et al [7]. described the dermoscopic features of acral melanoma in situ (n=25) and reported that the most common dermoscopic pattern was an asymmetric pattern (88%), followed by the PRP (84%), irregular dots and globules (40%), irregular blotches (24%), regression and irregular fibrillar pattern (each, 8%), ulcer, blue-white veil, and atypical vascular patterns (each, 4%), and polychromia (0%). We believe that the present results and those of Mun et al [7] could help differentiate ambiguous cases between acral melanoma and SH. In other words, we diagnosed SH if polychromia was noticed; vice versa, acral melanoma was presumed to be present when blue-white veils, regression structures, atypical vascular patterns, or irregular fibrillar patterns were found. Among the various dermoscopic features of acral melanoma, PRP is the most prominent [12]. Thus, most dermoscopists tend to diagnose acral melanoma when observing PRP, although PRP can be found in SH, lentiginosis, racial melanosis, acral melanocytic nevi, drug-induced hyperpigmentation, and dye-related pigmentation [13]. Therefore, we need to know how PRP differs between SH and acral melanoma. More than half of the PRP cases in this study showed pebbles on the ridges, which is the most characteristic hematoma-associated dermoscopic pattern. In the 12 cases without pebbles on the ridges, the color of PRP was red (n=3), black (n=3), or brown (n=6). Considering that the color of PRP is black or brown in acral melanoma, nine cases in this study showed a PRP similar to that of acral melanoma. However, four cases of brown PRP showed a brick wall-like structure, which has not been reported for acral melanoma. The remaining five cases with black or brown PRP (5/26, 19.2%) were similar to acral melanoma-associated PRP. Taking these results into account, the PRP of SH is quite different from that of acral melanoma, as SH-associated PRP presents with pebbles on the ridges, a red hue, and a brick wall-like structure. As we considered the bruise color sign to be a potentially meaningful dermoscopic feature of SH, we analyzed the dermoscopic patterns in the seven cases without the bruise color sign. They were composed of a brown to black homogenous pattern (n=3), brown PRP with a brick wall-like structure (n=2), red PRP (n=1), and red irregular dots and globules (n=1), which were not relevant to the dermoscopic findings of acral melanoma. The limitations of our study could be its retrospective design and small sample size, although this was the largest sample reported so far in the literature. In conclusion, we found and proposed some meaningful results for the features of SH and the dermoscopic differences between SH and acral melanoma. The exact ratio of the typical dermoscopic features of SH, acral nevus, and acral melanoma-associated patterns were 60%, 0%, and 72%, respectively. Although more than half of SH (26/50, 52%) cases could have PRP, the PRP seen in SH had pebbles on the ridges, a red hue, and a brick wall-like structure, which were not reported in PRP seen in acral melanoma. As new findings, we coined a new term, the bruise color sign, which could be a characteristic feature of SH and useful for discriminating SH from acral melanoma. Furthermore, we propose that a presumptive diagnosis of SH can be made if polychromia is noticed; vice versa, acral melanoma can be diagnosed when blue-white veils, regression structures, atypical vascular pattern, or irregular fibrillar patterns are found. We believe that the results of this study are very useful for dermatologists encountering ambiguous cases between SH and acral melanoma.
Acknowledgments
The patients in this manuscript have given written informed consent to publication of their case details.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Conflicts of Interests
The authors declare that there are no conflicts of interest.
References:
- Zalaudek I, Argenziano G, Soyer HP, et al. Dermoscopy of subcorneal hematoma. Dermatol Surg 30 (2004): 1229-1232.
- Elmas OF, Akdeniz N. Subcorneal hematoma as an imitator of acral melanoma: Dermoscopic diagnosis. North Clin Istanb 7 (2020): 56-59.
- Uslu U, Heppt F, Erdmann M. Intracorneal Hematoma Showing Clinical and Dermoscopic Features of Acral Lentiginous Melanoma. Case Rep Dermatol Med 2017 (2017): 3509146.
- Nigam M, Saxena D, Mishra PK, et al. Assessment of the age of bruise by their healing. Indian J Forensic Community Med 5 (2018): 119-122.
- Saida T, Oguchi S, Miyazaki A. Dermoscopy for acral pigmented skin lesions. Clin Dermatol 2002;20:279-85.
- Ozdemir F, Karaarslan IK, Akalin T. Variations in the dermoscopic features of acquired acral melanocytic nevi. Arch Dermatol 143 (2007): 1378-1384.
- Mun JH, Jo G, Darmawan CC, et al. Association between Breslow thickness and dermoscopic findings in acral melanoma. J Am Acad Dermatol 79 (2018): 831-835.
- Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol 48 (2003): 679-693.
- Savoia F, Ravaioli GM, Tabanelli M, et al. Scraping test for the diagnosis of acral subcorneal hemorrhage. J Am Acad Dermatol 81 (2019): 29-30.
- Kaminska-Winciorek G, Spiewak R. Tips and tricks in the dermoscopy of pigmented lesions. BMC Dermatol 12 (2012): 14.
- Braun RP, Thomas L, Dusza SW, et al. Dermoscopy of acral melanoma: a multicenter study on behalf of the international dermoscopy society. Dermatology 227 (2013): 373-380.
- Darmawan CC, Jo G, Montenegro SE, Kwak Y, et al. Early detection of acral melanoma: A review of clinical, dermoscopic, histopathologic, and molecular characteristics. J Am Acad Dermatol 81 (2019): 805-812.
- Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. A Bras Dermatol 88 (2013): 646-648.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 