Asymmetrical Cerebral Lateral Ventricles in Mild Cognitive Impairment and Alzheimer Disease
Article Information
Amanda Farah Khan1,2, Robert Bartha1, Cedric Annweiler1,3,4*
1Imaging Research Laboratories, Robarts Research Institute, Department of Medical Biophysics, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
2MD/PhD Program, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
3Department of Geriatric Medicine, Angers University Hospital; Angers University Memory Clinic; Research Center on Autonomy and Longevity, CeRAL; UNIV ANGERS, UPRES EA 4638, University of Angers, Angers, France
4Gérontopôle des Pays de la Loire, Nantes, France
*Corresponding Author: Cedric Annweiler, MD, PhD, Department of Geriatric Medicine, Angers University Hospital, 49933 Angers Cedex 9, France.
Received: 07 January 2024; Accepted: 11 January 2024; Published: 19 March 2024
Citation: Amanda Farah Khan, Robert Bartha, Cedric Annweiler. Asymmetrical Cerebral Lateral Ventricles in Mild Cognitive Impairment and Alzheimer Disease. Archives of Clinical and Biomedical Research. 8 (2024): 106-111.
View / Download Pdf Share at FacebookAbstract
Background: Growing evidence suggests a right-less-than-left asymmetry in pathological process during the course of Alzheimer disease (AD) between brain hemispheres. The purpose of this study was to investigate the left versus right volumes of the lateral ventricles, an aggregate measure of brain matter loss, in older adults with normal cognition, mild cognitive impairment (MCI; i.e., prodromal AD) and AD.
Methods: We studied 96 community-dwelling older participants (mean, 72.3±6.2years; 55.2% women) followed in the Memory Clinic of Angers University Hospital, France, as part of the GAIT study. Patients had their lateral ventricles volume quantified using semi-automated software from three-dimensional T1-weighted MRI.
Results: We found a left-right asymmetric enlargement of the lateral ventricles in participants with MCI and AD, but not in cognitively healthy individuals. This asymmetry involved the main body volumes, but not the temporal horns.
Conclusions: Our results add support to the assumption of an asymmetry of pathology in AD. Asymmetrical atrophy may serve as a potential biomarker of AD useful to discriminate between cognitively healthy individuals and those with AD.
Keywords
Alzheimer disease; Brain lateral ventricles; Magnetic resonance imaging; Older adults; Volumetry
Alzheimer disease articles; Brain lateral ventricles articles; Magnetic resonance imaging articles; Older adults articles; Volumetry articles
Article Details
Introduction
Alzheimer disease (AD) is the most frequently diagnosed degenerative dementia, affecting more than 26 million people worldwide [1]. Specific neuropathological changes associated with the disease have been reported in post-mortem studies, including intracellular tau-associated neurofibrillary tangles and extracellular amyloid-β plaques [2]. These pathological insults result in neuronal death and subsequent brain atrophy [3]. Generalized brain atrophy occurs in normal older adults as well, but this age-related loss of approximately 0.5-1% of brain volume per year after the age of 60 [4] is of a lower magnitude compared to the 2-percent loss observed in patients with AD every year [5]. Recent developments in neuroimaging research allow us to measure with unprecedented accuracy from magnetic resonance imaging (MRI) neuroanatomical morphological changes in the brain. This can be achieved by performing either manual tracing or using semi-automated or fully-automated volumetric software program that quantify the volume of the whole brain or the subvolumes of different brain regions [6]. In particular, bilateral medial temporal lobe atrophy has been reported in normal aging but also throughout the course of AD [2, 7-10]. The extent of cortex volume loss develops gradually from the prodromal stage of AD (i.e., mild cognitive impairment, MCI) [11] to the more severe stages of AD [2, 12-14], and is predictive of future cognitive and functional declines [12, 15]. Importantly, growing evidence suggests that the pathology of MCI and AD may not be symmetrical between brain hemispheres [12], with subsequent risk of asymmetrical atrophy [7]. Specifically, a left lateralisation of brain atrophy has been reported [5,7,11], despite conflicting results [16, 17]. The potential relevance of such asymmetrical atrophy in AD, if confirmed, would be that it could serve as a potential biomarker of AD useful to discriminate between healthy individuals and those with AD [7]. A simple, fast and automatic mechanism to explore brain matter volume is to measure the adjacent subvolumes of the lateral cerebral ventricles for differential changes [18-20]. Indeed, cerebrospinal fluid is under pressure and any parenchymal loss results in passive ventricle fluid expansion [21]. Modern software can segment ventricles volumes into subvolumes, which provides further insight into the volume of specific adjacent brain structures [19, 22, 23]. For instance, atrophy of medial temporal lobe leads to temporal horn enlargement [24]. We hypothesized that there could be a right-less-than-left asymmetry in lateral ventricle volumes during the course of AD. The purpose of this study was to investigate the left versus right volumes of the lateral ventricles in cognitively healthy individuals (CHI) and participants with MCI and AD. Our secondary objective was to compare the subvolumes of the left and right main bodies and temporal horns.
Materials and Methods
Participants
We studied 96 community-dwelling older participants (mean age 72.1±6.4years; 54.3% women) followed in the Memory Clinic of the University Hospital of Angers, France, from November 2009 to June 2011. All patients presented at the Clinic for a subjective memory complaint and were recruited after having given their written informed consent to the Gait and Alzheimer Interactions Tracking (GAIT) study. The GAIT study is an observational cohort study designed to examine gait in older adults with a subjective memory complaint [25]. Subjective memory complaints were documented using the Subjective Memory Complaints Questionnaire [26] and exclusion criteria included: age <60 years, Mini-Mental State Examination (MMSE) score <10 [27], the inability to walk independently, a history of stroke, a history of acute medical illness within the past 3 months, current delirium, severe depression and the inability to understand or answer the study questionnaires. All study participants received a thorough medical examination that consisted of structured questionnaires, a standardized clinical examination and a cerebral MRI evaluation. The study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983) and the protocol was approved by the University of Angers Ethical Review Committee (CPP Ouest II - 2009-12).
Diagnoses of CHI, MCI and AD
The diagnoses of MCI and AD were made during multidisciplinary meetings involving geriatricians, neurologists and neuropsychologists from the Memory Clinic of Angers University Hospital, and were based on neuropsychological testing, physical examination findings, blood tests and MRI of the brain. Neuropsychological assessment was performed during a face-to-face examination carried out by a neuropsychologist. The following standardized tests were used to probe several aspects of cognitive function: MMSE [28], Frontal Assessment Battery (FAB) [29], Alzheimer Disease Assessment Scale-cognitive subscale (ADAS-cog) [30], Trail Making Test (TMT) parts A and B [31], the French version of the Free and Cued Selective Reminding Test [32] and the Instrumental Activities of Daily Living scale (IADL) [33]. MCI was diagnosed according to Winblad et al. consensus criteria [34]. Participants with all categories of MCI were included in this study, i.e. amnesic and non-amnesic, as well as single and multiple affected domains. The diagnosis of AD followed the DSM-IV and NINCDS/ADRDA criteria [35]. Participants who were neither MCI nor AD and who had normal neuropsychological and functional performance were considered to be CHI.
Lateral ventricles volumetry
Imaging of the brain was performed with a 1.5 Tesla MRI scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) using a standardized MRI protocol that included images acquired with an axial T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) sequence, an axial fluid-attenuated inversion recovery (FLAIR) sequence, an axial turbo spin-echo (TSE) sequence, and a transverse T2* pulse sequence. Measurements of lateral ventricles volume were made from the raw 3D T1-weighted MP-RAGE images (acquisition matrix = 256x256x144, FOV= 240mm x 240mm x 187mm, TE/TR/TI = 4.07ms/2170ms/1100ms). All volumetric analyses were performed on a PC workstation running the Windows XP 64-bit operating system, using a software suite called Brain Ventricle Quantification (BVG; Merge Healthcare, Mississauga, ON). BVQ is a semi-automated volumetric analysis package designed to identify, segment and quantify lateral ventricles volume and subvolumes using T1-weighted MRI images of the brain. The algorithm behind BVQ has been previously reported in literature [19, 20]. In summary, four user-selected seed points were placed in each lateral ventricle and a region-growing algorithm automatically expanded the seed points within the 3D space of the image to the margin of the periventricular tissue. A volume was automatically calculated from identified voxels. Lastly, the falx cerebri as a landmark was automatically used to split the ventricle into left and right hemispheres, and the posterior commissure was used to separate the main ventricular bodies and the temporal horns (Figure 1). A single researcher (CA), who was blinded to age, sex and all clinical and biological information, performed all volumetric analyses. The measurement of whole ventricle volume is highly reproducible with intra- and inter-operator correlation coefficients greater than 0.98 [19]. Normalization of volumes to other brain structures and to whole brain volume was not performed as it has previously been demonstrated that this normalization does not significantly affect results [19, 36].
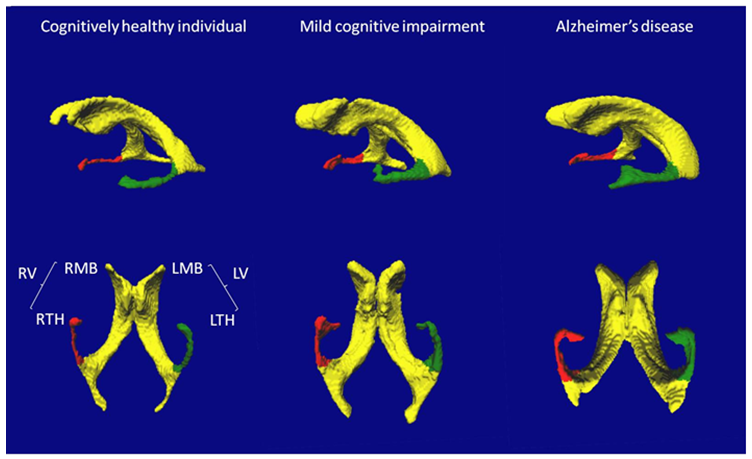
Figure 1: Representative examples of 3D-rendered lateral and inferior views of lateral cerebral ventricles in a cognitively healthy individual, in a participant with Mild Cognitive Impairment (MCI) and in a participant with Alzheimer disease (AD). The labelled volumes are the left (L) and right (R) lateral ventricles (V), main bodies (MB), and temporal horns (TH).
The RV and LV were significantly larger in MCI and AD compared to CHI, and the LV was larger than the RV in MCI and AD.
Statistical Analysis
All statistical analyses were performed with SPSS (v19.0, IBM Corporation, Chicago, IL). Means and standard deviations (SD) were calculated for demographic data and lateral ventricles volumes and subvolumes (i.e., main bodies and temporal horns). An analysis of variance (ANOVA) was performed on the 3 groups (i.e., CHI, MCI, AD) to compare left and right lateral ventricles volumes and subvolumes. Ad-hoc multiple comparisons were performed with a Bonferroni adjustment for each subsequent analysis. Finally, paired Student’s t-test was performed to compare left and right lateral ventricles volume and left and right lateral temporal horn volumes within each group.
Results
Among the 96 older adults included in this analysis (mean age, 72.3±6.2 years; 55.2% women) the mean lateral ventricle volume was 40.3 mL (range: 13.8 mL-154.6 mL). Thirty-eight participants were CHI (mean age, 69.7±3.6 years; 60.5% women), 35 were diagnosed with MCI (mean age, 69.7±3.7 years; 45.7% women) and 23 with AD (mean age, 80.6±5.3 years; 60.9% women). As illustrated in Tables 1 and 2, a trend for larger lateral ventricles was found between participants with AD compared to those with MCI (51.5 mL versus 38.6 mL on average; P= 0.056). The total volume of lateral ventricles did not differ significantly between CHI and MCI (35.0 mL versus 38.6 mL respectively, P= 1.00). In particular, there were no differences regarding the subvolumes of the main bodies and of the temporal horns between CHI and those with MCI groups (P > 0.05 for both measures). In contrast, the AD group had larger lateral ventricles, main bodies and temporal horns than the CHI (P < 0.05 for all measures). Lastly, only the temporal horns, but not the main bodies, were significantly larger in people with AD compared to those with MCI (P = 0.009 for left temporal horn subvolume, and P = 0.007 for right temporal horn subvolume).
Table 1: Participants’ characteristics.
|
Groups |
Total volume of lateral ventricles |
Subvolume of left lateral ventricle |
Subvolume of right lateral ventricle |
Subvolume of left temporal horn |
Subvolume of right temporal horn |
|
CHI – MCI |
P = 1.00 |
P = 1.00 |
P = 1.00 |
P = 1.00 |
P = 1.00 |
|
CHI – AD |
P = 0.008* |
P = 0.004* |
P = 0.022* |
P = 0.016* |
P = 0.019* |
|
MCI – AD |
P = 0.056 |
P = 0.050 |
P = 0.081 |
P = 0.009* |
P = 0.007* |
Table 2: ANOVA inter-group results (significance reported).
As illustrated in Table 3, CHI had no differences between the left and right volumes of the lateral ventricles (P = 1.70), of the main ventricular bodies (P = 0.163) and of the temporal horns (P = 0.329). Conversely, in the group with MCI, we found a left-right asymmetry for the volume of the lateral ventricles illustrated by a larger left lateral ventricle (P = 0.004) and a larger left main ventricular body (P = .008), but no volumic difference between the temporal horns (P = 0.329). Finally, in the group with AD, we also found larger left lateral ventricles (P = 0.016) and larger main ventricular bodies (P = 0.028), but no asymmetry in the temporal horns subvolumes (P = 0.918).
|
Groups |
Subvolumes of left and right lateral ventricle |
Subvolumes of left and right main bodies of lateral ventricles |
Subvolumes of left and right temporal horns |
|
CHI |
1.4 (P = 1.70) |
1.4 (P = 0.163) |
0.99 (P = 0.329) |
|
MCI |
3.1 (P = 0.004)* |
2.8 (P = 0.008)* |
0.32 (P = 0.748) |
|
AD |
2.6 (P = 0.016)* |
2.4 (P = 0.028)* |
0.104 (P = 0.918) |
Table 3: Paired samples Student’s t-test results (t-value and significance reported).
Discussion
The main finding of this memory clinic-based study is that there was a right-less-than-left asymmetric enlargement of the lateral ventricles among participants with MCI and AD. We described two different profiles of expansion: first, when comparing CHI and MCI, we found no significant enlargement of the ventricles but a left enlargement of the main ventricular body compared to the right one in MCI specifically; and then, when comparing MCI and AD, we found a global enlargement of the whole ventricles (notably of the subvolume of temporal horns) with a persistent circumscribed asymmetric enlargement of the left main ventricular body. In contrast, we found no asymmetry of the left and right temporal horns in the 3 groups. The results of this study add support to the hypothesis that there may be an asymmetry of pathology in patients with AD and MCI. As previously reported by Muller et al., there may be a noteworthy functional significance to this finding [37]. In their study, left hippocampal tissue breakdown was the strongest single predictor of verbal memory performance, as atrophy in this area of the brain seemed to be associated with impaired encoding and retrieval of verbal material that occurs early on in the course of AD [37]. Shi et al., further expanded on the importance of the left vs. right asymmetry by suggesting that the asymmetry may change dynamically with disease progression [9]. Apostolova et al. [7] reported that participants with MCI who had moderate to severe hippocampal atrophy showed a trend for greater left-sided hippocampal atrophy versus the right. Thompson et al. [14] showed that cortical atrophy occurred earlier and progressed faster in the left hemisphere among participants with AD on average. This asymmetry was further confirmed in a meta-analysis by Shi et al. (2009) that was performed in controls, MCI and AD patients. This group found that consistent left-less-than-right asymmetry within all three cohorts. When using the right hippocampus as a baseline measurement, the relative left hippocampus average volume reduction, weighted by sample size was 6.3% (SD 4.9%) in patients with Alzheimer disease, 9.1% (SD 6.5%) in patients with Mild Cognitive Impairment, and 5.8% (SD 4.6%) in controls [9]. The highest disturbance in hippocampal asymmetry occurred in MCI populations and Shi et al. suggests that this may be a characteristic of the onset of illness. Lastly, Giannakopoulos et al. investigated the relationship between microvascular pathology in the left and right hemispheres of the brain in 153 individuals whose brain was examined post-mortem [38]. They found that many more brains presented with a predominance of microvascular pathology in the left hemisphere than right. Additionally, they discovered that cognitive status was positively associated with Alzheimer and microvascular pathology in each hemisphere. Bugiani et. al also found similar findings at autopsy, noting that atrophy of the brain was more severe in the association areas surrounding the left sylvian fissue and that numerical densities of nerve cells were lower in the left hemisphere [17].
Limitations of this study include the fact that it is cross-sectional in design and did not follow patients longitudinally to track ventricles volume expansion over time. Future studies can be designed with multiple imaging and cognitive testing time points. Thus, the disturbance of hippocampal symmetry may be a characteristic biomarker of the onset of illness and can be used to perhaps discriminate between healthy individuals and those with AD pathology. Additional imaging studies need to be performed to confirm and further explore the asymmetry of pathology and Alzheimer.
Acknowledgments
The authors have listed everyone who contributed significantly to the work in the Acknowledgments section. Permission has been obtained from all persons named in the Acknowledgments section.
Melinda Beaudenon, MS, Jennifer Gautier, MS, and Romain Simon, MS, from Angers University Memory Clinic, France, for daily assistance. There was no compensation for this contribution.
Jean-Yves Tanguy, MD, Anne Pasco-Papon, MD, PhD, Christophe Aube, MD, PhD, from Department of Radiology, Angers University Hospital, France, for technical support. There was no compensation for this contribution.
Irene B Gulka, MD, from the Department of Radiology, London Health Sciences Centre, London, ON, Canada, for her kind advice. There was no compensation for this contribution.
Conflict of Interest
Disclosures:
AFK: reports no disclosures. She has no relevant financial interest in this manuscript.
RB: founding part-owner of Bioscape Medical Imaging Inc. He has no relevant financial interest in this manuscript.
CA: reports no disclosures. He has no relevant financial interest in this manuscript.
Funding
The study was financially supported by the French Ministry of Health (Projet Hospitalier de Recherche Clinique national n°2009-A00533-54).
Software for quantification of brain ventricle volumes was provided by Merge Healthcare (Mississauga, ON, Canada).
Dr. Khan supported by a grant from the Canadian Institutes for Health and Research (CIHR) (MD/PhD Studentship) and a grant from the McLaughlin Centre at the University of Toronto (Studentship).
Dr. Annweiler supported by a grant from the CIHR - Institute of Aging.
The sponsors had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Authors Contributions
Dr. Annweiler has access to all the study data, takes responsibility for the accuracy of the analysis, and has authority over manuscript preparation and the decision to submit the manuscript for publication.
Study concept and design
Khan and Annweiler
Acquisition of data
Annweiler
Analysis and interpretation of data
Khan and Annweiler
Drafting of the manuscript
Khan and Annweiler
Critical revision of the manuscript for important intellectual content
Bartha.
Statistical analysis
Annweiler
Obtaining funding
Annweiler
Administrative, technical, or material support
Annweiler
Supervision
Annweiler
References
- Ferri CP, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 366 (2005): 2112-2117.
- Sabuncu MR, et al. The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch Neurol 68 (2011): 1040-1048.
- Chintamaneni M, M Bhaskar. Biomarkers in Alzheimer's Disease: A Review. ISRN Pharmacol 2012 (2012): 984786.
- Fjell AM, et al. One-year brain atrophy evident in healthy aging. J Neurosci 29 (2009): 15223-15231.
- Sluimer JD, et al. Whole-brain atrophy rate in Alzheimer disease: identifying fast progressors. Neurology 70 (2008): 1836-1841.
- Shen Q, et al. Volumetric and visual rating of magnetic resonance imaging scans in the diagnosis of amnestic mild cognitive impairment and Alzheimer's disease. Alzheimers Dement 7 (2011): e101-108.
- Apostolova LG, et al. 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp 31 (2010): 786-797.
- Cherbuin N, et al. Mild Cognitive Disorders are Associated with Different Patterns of Brain asymmetry than Normal Aging: The PATH through Life Study. Front Psychiatry 1 (2010): 11.
- Shi F, et al. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus 19 (2009): 1055-1064.
- Raz N, et al. Relationship between cognitive and morphological asymmetry in dementia of the Alzheimer type: a CT scan study. Int J Neurosci 35 (1987): 225-232.
- Simon SS, JE Yokomizo, CM Bottino. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev 36 (2012): 1163-1178.
- Jack CR Jr, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62 (2004): 591-600.
- Hsu YY, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging 16 (2002): 305-310.
- Thompson PM, et al. Tracking Alzheimer's disease. Ann N Y Acad Sci 1097 (2007): 183-214.
- Sluimer JD, et al. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology 248 (2008): 590-598.
- Derflinger S, et al. Grey-matter atrophy in Alzheimer's disease is asymmetric but not lateralized. J Alzheimers Dis 25 (2011): 347-357.
- Bugiani O, et al. Asymmetrical cerebral atrophy in Alzheimer's disease. Clin Neuropathol 10 (1991): 55-60.
- Annweiler C, et al. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 78 (2012): 1281.
- Nestor SM, et al. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain 131 (2008): 2443-2454.
- Khan AF, et al. A novel MRI-compatible brain ventricle phantom for validation of segmentation and volumetry methods. J Magn Reson Imaging 36 (2012): 476-482.
- Bradley WG Jr. Magnetic resonance imaging in the evaluation of cerebrospinal fluid flow abnormalities. Magn Reson Q 8 (1992): 169-196.
- Schott JM, et al. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology 65 (2005): 119-124.
- Jack CR Jr, et al. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement 6 (2010): 212-220.
- Chance SA, MM Esiri, TJ Crow. Ventricular enlargement in schizophrenia: a primary change in the temporal lobe? Schizophr Res 62 (2003): 123-131.
- Annweiler C, et al. Vitamin D insufficiency and mild cognitive impairment: cross-sectional association. Eur J Neurol 19 (2012): 1023-1029.
- Youn JC, et al. Development of the Subjective Memory Complaints Questionnaire. Dement Geriatr Cogn Disord 27 (2009): 310-317.
- Tombaugh TN, NJ McIntyre. The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society (1992).
- Folstein MF, SE Folstein, PR McHugh. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12 (1975): 189-198.
- Dubois B, et al. The FAB: a Frontal Assessment Battery at bedside. Neurology 55 (2000): 1621-1626.
- Rosen WG, RC Mohs, KL Davis. A new rating scale for Alzheimer's disease. Am J Psychiatry 141 (1984): 1356-1364.
- Brown EC, et al. Trial making test as a screening device for the detection of brain damage. J Consult Psychol 22 (1958): 469-474.
- Grober E, et al. Screening for dementia by memory testing. Neurology 38 (1988): 900-903.
- Peres K, et al. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 67 (2006): 461-466.
- Winblad B, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256 (2004): 240-246.
- McKhann G, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34 (1984): 939-944.
- Carmichael OT, et al. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging 28 (2007): 389-397.
- Muller MJ, et al. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage 28 (2005): 1033-1042.
- Giannakopoulos P, et al. Interhemispheric distribution of Alzheimer disease and vascular pathology in brain aging. Stroke 40 (2009): 983-986.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 