Fatal Palbociclib -Related Interstitial Pneumonitis
Article Information
Levy Ofer MD1*, Ptashkin Ekaterina MD1, Shechtman Yitzhak MD2, Kas, Tamar MD3, Natif Noam MD4, Sanset Sofia MD1, Katz Daniela MD5
1Department of Medicine B, Shamir Medical Center, Zrifin, Beer Yaacov. Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel
2Department of Pathology, Shamir Medical Center, Zerifin, Beer Yaacov. Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel
3Department of Diagnostic Imaging, Shamir Medical Center, Zerifin, Beer Yaacov, Israel
4Pulmonary Institute, Shamir Medical Center, Zrifin, Beer Yaacov. Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel
5Institute of Oncology, Shamir Medical Center, Zrifin, Beer Yaacov, Israel
*Corresponding Author: Dr. Levy Ofer MD, Department of Medicine B, Shamir Medical Center, Zrifin, Beer Yaacov. Sackler faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel
Received: 05 June 2019; Accepted: 11 June 2019; Published: 12 August 2019
Citation: Levy Ofer MD, Ptashkin Ekaterina MD, Shechtman Yitzhak MD, Kas, Tamar MD, Natif Noam MD, Sanset Sofia MD, Katz Daniela MD. Fatal Palbocyclib-Related Interstitial Pneumonitis. Archives of Clinical and Medical Case Reports 3 (2019): 162-166.
View / Download Pdf Share at FacebookAbstract
Palbociclib is indicated in combination with an aromatase inhibitor for first line treatment of hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer. We report a case of a 71-year-old patient with metastatic breast cancer who developed fatal diffuse parenchymal lung injury three months after the initiation of palbociclib plus letrozole. The patient presented with pulmonary embolism, which masked the progression of diffuse parenchymal lung injury. As dyspnea worsened, chest CT revealed a mixed pattern of severe interstitial and alveolar lung injury. Transbronchial lung biopsy was consistent with the subacute lung injury pattern. Treatment with high dose glucocorticoids did not result in clinical improvement and the patient succumbed to respiratory failure. It is important for clinicians to be aware that fatal interstitial pmeumonitis may occur in patients who are treated with palbociclib because early diagnosis may be vital.
Keywords
Drug-induced pneumonitis, Palbociclib, Pulmonary embolism, Breast cancer
Article Details
1. Introduction
The lung is certainly one of the target organs for drug-related adverse effects, and diffuse parenchymal lung disease is a particularly important manifestation of drug toxicity. It is imperative that drug toxicity be considered in all patients who develop interstitial lung disease, particularly under chemotherapeutic or cytotoxic agents [1]. In general, the risk of developing diffuse parenchymal lung disease is increased with higher cumulative doses of a particular agent, but occasional cases with even relatively low cumulative doses are described. In most cases, diffuse parenchymal lung disease develops during a period ranging from 1 month to several years after use of the agent, but some agents can also be associated with the development of more acute disease. For patients who are believed to have drug-related diffuse parenchymal lung disease, the particular agent ideally should be discontinued. Steroids may be administered, but the results are variable [2].
Palbociclib is indicated for the treatment of HR positive, HER2 negative advanced or metastatic breast cancer in combination with letrozole as initial endocrine based therapy in postmenopausal women [3]. Adverse reactions of interstitial lung disease (ILD)/non-infectious pneumonitis have been identified during post-approval use of palbociclib, Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Two serious pulmonary adverse events were reported in clinical trials of other cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitors. One death due to pneumonitis occurred with abemaciclib plus anastrozole or letrozole in the MONARCH 3 study [4]. Another death due to acute respitratory distress syndrome occurred with ribociclib plus fulvestrant in the MONALEESA-3 trial [5]. To our knowledge, we report the first case of palbociclib-related diffuse parenchymal lung injury.
2. Case Report
In January 2010, a 63-year-old women, presented with inflammatory carcinoma of breast ER/PR+, HER2-. Neoadjuvant chemotherapy with dose dense doxorubicin in combination with cyclophosphamide followed by paclitaxel was initiated. Lumpectomy plus sentinel lymph node biopsy were performed 6 months later. After the completion of adjuvant radiotherapy, adjuvant hormonal therapy with tamoxifen followed by exemestane were administrated for a total of 5 years until September 2015. Two years later, the patient was diagnosed with metastatic breast cancer following progressive diffuse bone pain. Palliative radiotherapy to the lumbar spine and pelvis was administrated and treatment with letrozole, palbociclib and zoledronic acid was initiated in April 2018.
In early June 2018, the patient reported her bone pain improved, the level of CA-15.3 decreased (from 205 to 76 units per milliliter) and PET-CT was consistent with partial response and without evidence of any lung pathology. Ten days later, the patient was admitted to an internal medicine ward due to shortness of breath and swelling of the left leg. Doppler ultrasound revealed an acute deep venous thrombosis of the left distal femoral vein, and rivaroxaban was started.
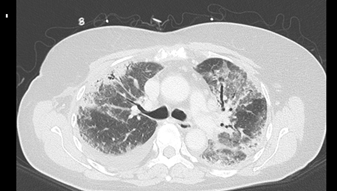
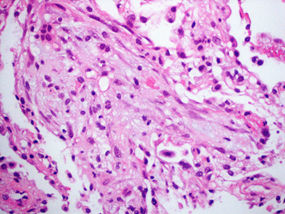
Two weeks later the patient was readmitted due to dyspnea. CT pulmonary angiogram demonstrated a saddle-type pulmonary embolus extending bilaterally into the pulmonary arteries. In addition, mild peripheral signs of interstitial lung diseases were noted. Anticoagulation therapy was switched to enoxaparin on admission. During her hospitalization, dyspnea continued. Echocardiography was unremarkable for right ventricular dysfunction and anti-Xa level was in therapeutic range. On the 10th day of admission, piperacillin/tazobactam (Tazocin) was initiated due to a new left lower lobe infiltrate on chest x-ray suspected for hospital acquired pneumonia. Four days later, dyspnea progressed and the patient developed bilateral respiratory rales and hypoxemia. White Blood cell count was 3.9 K/µl, neutrophils 2.7 K/µl, Hgb 10.1 g/dl, platelets 196 K/µl. Hepatic and renal tests were normal. C reactive protein level was 84 mg/mL and procalcitonin level was 0.31 ng/ml (0.0-0.5). Chest CT on 15th day of admission revealed new ground glass opacities and interlobar septal thickening in the upper lung fields, diffuse alveolar infiltrates in the lower lung fields, and bilateral pleural effusion (Figure 1). Bronchoscopy and bronchial alveolar lavage were performed. Blood and sputum cultures remained sterile and a comprehensive infectious disease workup was negative for urinary Legionella pneumophila antigen, Streptococcus pneumonia antigen, respiratory viruses PCR panel (Adenovirus, Enterovirus, Parainfluenza, and Human metapneumovirus), bacterial sputum PCR panel (Mycoplasma pneumonia, Legionella pneumophila, Streptococcus pneumonia, Hemophilus influenza, Bordetella pertussis, Chlamydia pneumoniae, Bordetella parapertusis), PCR for Pneumocystis jirovecii and PCR for Mycobacterium tuberculosis. Transbronchial lung biopsy was consistent with subacute interstitial lung injury, without evidence of malignancy (Figure 2).
The patient was transferred to an intensive care unit due to respiratory failure. Treatment with wide spectrum antibiotics and high dose glucocorticoids did not result in clinical or radiological improvement and the patient succumbed three weeks later.
3. Discussion
This patient presented with diffuse parenchymal lung disease three months after palbociclib, letrozole and zoledronic acid were initiated and proved efficacious on PET CT. Lung injury was confirmed on sequential CT imaging studies. No previous cases of letrozole or zoledronic acid related pneumonitis have been reported. Alternative causes for diffuse parenchymal lung disease such as infection, pulmonary infarction, or lymphangitic carcinomatosis was excluded in an extensive laboratory workup and in transbronchial biopsy. The exclusion of these multiple diagnoses suggests a causal relationship to drug exposure. The Naranjo nomogram for adverse drug reaction assessment score for this case is 6. To our knowledge, this is the first case of palbociclib-induced pneumonitis to be reported.
Pulmonary embolism has been reported at a higher rate in patients treated with palbociclib plus letrozole (4%) compared to patients treated with letrozole alone in the PALOMA-1 study [6]. Moreover, in real world experience, 11% incidence of venous thromboembolism was observed in 64 women with metastatic breast cancer treated with palbociclib [7]. These figures may suggest that palbociclib in combination with endocrine therapy poses increased risk for venous thromboembolism.
Our patient with advanced breast cancer, achieved a partial response with palbociclib plus letrozole, but concomitantly presented with venous thromboembolism and diffuse parenchymal lung disease. The thromboembolic event has masked the progression of lung toxicity, as patient' progressive dyspnea was attributed to pulmonary embolism. It is important for clinicians to be aware that fatal interstitial pmeumonitis may occur in patients who are treated with palbociclib because early diagnosis may be vital.
Disclosure
The authors have no conflicts of interest to disclose.
References
- Skeoch S, Weatherley N, Swift AJ, et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. J Clin Med 15 (2018): 7.
- Weinberger SE, Cockrill BA, Mandel J. Diffuse parenchymal lung diseases associated with known etiologic agents. In: Weinberger SE, Cockrill BA, Mandel J eds. Principles of Pulmonary Medicine, Elsevier, Inc. (2018): 145-157.
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 375 (2016): 1925-1936.
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5 (2019): 5.
- Slamon DJ, Neven P, Chia S, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol 36 (2018): 2465-2472.
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16 (2015): 25-35.
- Watson GA, Deac O, Aslam R, et al. Real-World Experience of Palbociclib-Induced Adverse Events and Compliance With Complete Blood Count Monitoring in Women With Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer. Clin Breast Cancer 19 (2019): e186-e194.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
