Acute Myeloid Leukemia with Platelet-Derived Growth Factor Receptor β (PDGFRβ) Rearrangement Successfully Treated with Intensive Chemotherapy in Combination Withimatinib- A Case Report
Article Information
Ewelina Witkowska1, 2, Katarzyna Jerzmanowska1, 2, Ewa Wawrzyniak1, 2, Marta Robak2, Agnieszka Wierzbowska1, 2, Agnieszka Pluta1, 2*
1Department of Haematology, Medical University of Lodz, Lodz, Poland
2Department of Haematology, Copernicus Provincial Multidisciplinary Oncology and Traumatology Centre, Lodz, Poland
*Corresponding Author: Agnieszka Pluta, Assistant Professor, Department of Haematology Medical University of Lodz, Copernicus Provincial Multidisciplinary Oncology and Traumatology Centre, ul. Ciolkowskiego 2, 93-510 Lodz, Poland
Received: 22 June 2021; Accepted: 15 July 2021; Published: 22 July 2021
Citation: Ewelina Witkowska, Katarzyna Jerzmanowska, Ewa Wawrzyniak, Marta Robak, Agnieszka Wierzbowska, Agnieszka Pluta. Acute Myeloid Leukemia with Platelet-Derived Growth Factor Receptor β (PDGFRβ) Rearrangement Successfully Treated with Intensive Chemotherapy in Combination Withimatinib- A Case Report. Archives of Clinical and Medical Case Reports 5 (2021): 559-566.
View / Download Pdf Share at FacebookAbstract
Acute myeloid leukemia (AML) is a heterogeneous clonal stem cell malignancy. Several subgroups of AML have been identified based on multiple genomic and proteomic aberrations; these demonstrate different clinical features, response to treatment and prognosis. Rearrangements in the platelet-derived growth factor receptor β (PDGFRβ) gene result in greater constitutive enzymatic activity (tyrosine kinase) and deregulation of haematopoiesis, similarly to BCR-ABL1 in chronic myeloidleukemia (CML). PDGFRβ gene rearrangements are infrequent entities, which are mostly diagnosed in patients presenting with atypical CML, chronic myelomonocytic leukemia (CMML), myelodysplastic/myeloproliferative disorders (MDS/MPN) or juvenile myelomonocytic leukemia (JMML). PDGFRβ-positive AML is extremely rare, accounting for well below 1% of all cases.
In this article, we present the case of a 31-year-old woman with AML and abnormal karyotype with PDGFRβ rearrangement, who was treated with intensive chemotherapy combined with low-dose imatinib. Complete remission (CR) was achieved and imatinib was used as maintenance treatment. The patient remains in CR with disease-free survival (DFS) of 41 months.
Keywords
Acute myeloid leukemia; PDGFRβ; Imatinib
Acute myeloid leukemia articles; PDGFR? articles; Imatinib articles
Article Details
1. Introduction
Acute myeloid leukemia (AML) is a malignant haematological disease characterized by an abnormal proliferation of myeloid progenitor cells that interferes with normal haematopoiesis [1]. The disease accounts for approximately 80% of adult acute leukemias and approximately 15% of childhood leukemias [1-3], with four new cases per 100000 people per year [1-3]. AML may affect people at any age, but most patients are over 65 at the time of diagnosis [4]. Acute myeloid leukemias are classified according to the presence of cytogenetic abnormalities and the corresponding fusion genes. The presence of such cytogenetic and molecular aberrations is of diagnostic importance, acts as an independent prognostic factor,and hence influences the choice of treatment [5]. The platelet-derived growth factor receptor (PDGFR) consists of two subunits, α and β, and acts as a protooncogene; it belongs to the receptor tyrosine kinase (RTK) family, together with various other transmembrane receptors including KIT receptor, colony stimulating factor 1 receptor (CSF1R) and Fms-like tyrosine kinase 3 receptor (FLT3) [6-8]. The platelet-derived growth factor ligands (PDGF-L): A, B, C, D and their receptors play a key role in proliferation, differentiation, growth and development of the cell, as well as in regulating the cell microenvironment [9, 10]. The activation of PDGFR results in inciting pathways that promote cell survival, such asmitogen-activated protein kinase (MAPK) or signal transducer and activator of transcription (STAT) [11]. Their abnormal expression plays an important role in many diseases, including cancer [10]; a high rate of PDGFR defects (more than 10%) have been reported in melanoma (~10–30%), lung (~10–20%), glioblastoma (~15–20%), bladder (15–20%), prostate (~20%), colorectal (~10–15%), and ovarian (~10–20%) cancers [11].
However, breast, liver and renal cancer as well as acute myeloid leukaemia and myeloproliferative disorders, are characterized by consistently lower incidences (<10%) [11, 12]. PDGFR gene rearrangements account for well below 1% of all cases of AML [12]. PDGFR mutations, including PDGFRα and PDGFRβ, are most often encountered in chronic myeloproliferative neoplasm (MPN) [12]: the incidence of PDGFRβ rearrangement cases in MPN is 1.8% [13].
2. Case Report
In June 2017, a 31-year-old woman was admitted to the Department of Hematology, Medical University of Lodz, for a diagnosis of hyperleukocytosis. The patient had previously been hospitalized for eight days in the Department of Infectious Diseases with a suspicion of mononucleosis due to fever, enlarged submandibular lymph nodes, massive sores on the tonsils and hepatomegaly. She complained of recurrent pharyngitis, four times in the previous six months, with no response to the administered treatment: cefuroxime and glucocorticosteroids. Finally, mononucleosis was excluded. AML was suspected, and the patient was admitted to the Hematology department. Upon admission, peripheral blood morphology revealed mild anaemia (haemoglobin of 10.8 g/dl, meancorpus-cularvolume (MCV) 91,7 fL), hyperleukocytosis (white blood cells 131.87G/L), thrombocytopenia (platelets 134G/L). A peripheral blood smear showed 50% blasts, 7% promyelocytes, 2% segmented neutrophils, 30% monocytes,10% lymphocytes and1% eosinophils. Notably, no eosinophilia or basophiliawas observed (Figure 1a). Bone marrow aspirate revealed 69% of myeloblasts (Figure 1b) without dysplastic features. Immunopheotypic analysis with eight-colour flow cytometry confirmed the myeloid origin of the blasts, with an expression of CD13 (32.4%), CD14 (15.7%), CD33 (96.8%), CD117 (14.7%), CD15 (88%), CD38 (87.4%), HLA-DR (82.2%), CD45 (99.3%).
Laboratory studies showed normal kidney function (creatinine 0.66 mg/dl; urea 28.7 mg/dl; uric acid 4.8 mg/dl; potassium 3.3 mmol/l; sodium 140 mmol/l), no signs of enzymaticliver damage (total bilirubin 0.2 g/dl; direct bilirubin 0.1 mg/dl; ALT 47 U/l; AST 31 U/l; GGTP 45 U/l), increased lactate dehydrogenase activity (LDH 543 U/l), hyperferritinemia (463.4 ng/ml), normal CRP values (2.50 mg/l). The following infections were excluded: hepatitis B virus (anty-HBs negative), hepatitis C virus (anty-HCV negative), human immunodeficiency virus (anty-HIV negative), Epstein-Barr Virus (anty-EBVIgM negative, anty-EBV IgG positive) and cytomegalovirus (anty-CMVIgM negative, anty-CMV IgG positive). Cytogenetic analysis of bone marrow cells performed at diagnosis revealed abnormal karyotype: 46, XX, t(2;5) (q32-33;q32) [11]/46, XX[3] with PDGFRβ (5q32-33.1) rearrangement confirmed by fluorescent in situ hybridization (FISH). Break Apart Probe (MetaSystems) was performed and showed PDGFRβ gen rearrangement in 90%of interphase nuclei and 8/10 metaphases. Additionally, painting probes for chromosome 2 and 5 were used to confirm balanced translocation t (2;5) (Figure 2).
The following diagnosis was thus established:de novoAML, NOS (not otherwise specified), 46, XX, t(2;5) (q32-33;q32) [11]/46,XX[3].ish(2;5) (5`PDGFRB+;3`PDGFRB+) [8/10].nucish (PDGFRBx2) (3`PDGFRBsep5`PDGFRBx1) [90/100], FLT3-ITD (negative), BCR-ABL1 (negative), WT1 (positive), MLL-PTD (negative), RUNX1-RUNX1T1 (negative), CBFB-MYH11 (negative), intermediate cytogenetic and molecular risk according to European LeukemiaNet classification (ELN 2017). The patient met the criteria for intensive chemotherapy and was offered standard induction treatment according to Polish Adult Leukemia Group (PALG) recommendations. A DAC (daunorubicin: 60 mg/m2, iv, days 1-3; cytarabine: 200 mg/m2, iv, days 1-7; cladribine: 5 mg/m2, iv, days 1-5) induction cycle was commenced [14, 15].
Before starting chemotherapy, the patient was diagnosed with superficial vein thrombosis in the left calf, which was treated with low molecular weight heparin. Doppler ultrasound showed complete regression of thrombotic changes in four weeks.
The post-treatment aplasia periodlasted 18 days and was complicated by fever up to 39oC with clinical and radiological features of bilateral pneumonia. Moreover, bacteraemia of methicillin-resistant Staphylococcus haemolitycus was diagnosed. Broad-spectrum and targeted antibiotic therapy (glycopeptides, carbapenems) was applied, resulting in improved general condition and pneumonia regression. During the period of aplasia, G-CSF (granulocyte colony-stimulating factor) was not administrated. Throughout hospitalization, the patient required a transfusion of six units of red blood cells and 20 units of platelets. Due to the PDGFRβ rearrangement, 100mg imatinib was administered once a day from the thirtieth day of the induction cycle. The hospitalization time was 35 days. Complete remission (CR) was achieved within 43 days. Between July and December 2017, the patient received three intensive consolidation courses (first course of cytarabine in a dose 2000 mg/m2 every 12 hours, iv, days 1-3; mitoxantrone: 10 mg/m2, iv, days 3-5, second and third cytarabine: 2000 mg/m2 every 12 hours, iv, days 1-3-5).
During each consolidation, imatinib was discontinued during the period of bone marrow aplasia for approximately 14 days. The patient was not qualified for allogeneic stem cell transplantation (allo-HCT) due to the lack of a sibling donor. The patient isin follow up in CR. Karyotype by conventional cytogenetics and PDGFRβby FISH remain normal after 44 months from diagnosis. Molecularminimal residual disease, PDGFRβ is also negative. The imatinib treatment (100 mg daily) is ongoing. Overall survival is 44 months, and disease-free survival is 42 months.
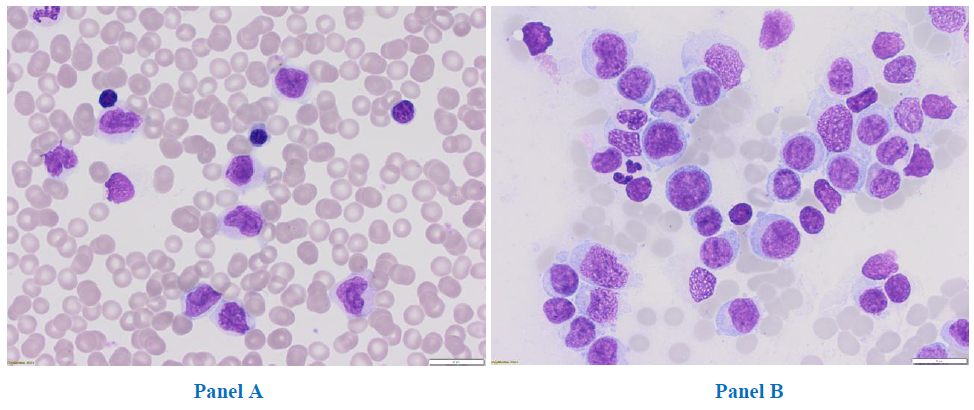
Figure 1: Peripheral blood, magnification 31,5x (Panel A) and bone marrow smear, magnification 31,5x (Panel B).
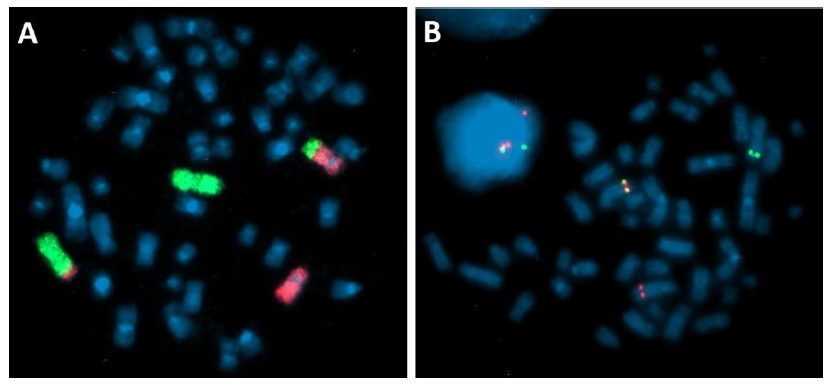
Figure 2: Fluorescence In Situ Hybridization on bone marrow cells after 24h culture. (A) Translocation t(2;5) (q32-33;q33). Painting probes: green – chromosome 2, red – chromosome 5. (B) PDGFRB rearrangement – Break Apart Probe: Fusion signal on normal chromosome 5, green signal on chromosome der(2)t(2;5), red signal on chromosome der(5)t(2;5).
3. Discussion
This report examined a case of t(2;5) (q32-33;q32) translocation resulting in a rearrangement of the PDGFRβ gene (5q32-33.1) in an AML patient. This kind of gene abnormality is extremally rare in patients diagnosed with AML [16]. Only a few cases of this AML subtype have been recorded to date. The fourth edition of the WHO classification, published 2008, introduced a new class of hematopoietic diseases: myeloid and lymphoid neoplasms with eosinophilia and abnormalities in PDGFRα, PDGFRβ and FGFR1 genes [17]. This list was supplemented with the PMC1-JAK2 aberration in 2016 [18]. The myeloid neoplasms characterised by PDGFRβ rearrangement have wider phenotypic and genotypic diversity than the neoplasms associated with PDGFRα rearrangement. For example, phenotypic changes have been observed in chronic eosinophilic leukemia, chronic myelomonocytic leukemia (usually with eosinophilia), atypical chronic myeloid leukemia (usually with eosinophilia), primary myelofibrosis, juvenile myelomonocytic leukemia with eosinophilia, AML (without eosinophilia), and chronic basophilic leukemia [19]. It has been reported that patients with PDGFRβ rearrangements, including those with AML, respond well to tyrosine kinase inhibitors such as imatinib [16]. Previous studies indicate that low-dose imatinib (100 mg/day) might have the same efficacy as higher dose imatinib (400 mg/day) in treating myeloid neoplasms with PDGFRβ abnormalities, such as atypical chronic myeloid leukemia [20].
The present study reports the case of a 31-year-old woman who was diagnosed with AML 46, XX, t(2;5) (q32-33;q32) with PDGFRβ rearrangement, stratified to the intermediate cytogenetic and molecular risk group according to European LeukemiaNet 2017 [21, 22]. The patient did not have an any HLA-matched sibling donor. She received standard induction and consolidation treatment combined with imatinib, which she is still receiving as maintenance treatment. A literature review revealed a couple of case reports, which were used to determine the preferred treatment for our patient. Chang et al. report the case of a 41-year-old man with concurrent AML and T lymphoblastic lymphoma with rearranged PDGFRβ gene. Their treatment strategy was based on cytarabine and daunorubicin, with steroids added in the induction phase. This patient achieved complete remission for both entities. He later underwent an allogeneic stem cell transplantation from an HLA-matched sibling donor; however, this was complicated by the development of severe chronic GVHD and death 11 months after the procedure. The authors recommend that tyrosine kinase inhibitors should be considered by clinicians coping with diseases connected to PDGFRβ rearrangement, and suggest that while allo-HCT is an efficient procedure, it is also a risky one, with many adverse events. Therefore the decision about alloHCT in cases involving PDGFRβ mutations should be made very carefully, especially when there is wide access to tyrosine kinase inhibitors (TKI) [23].
Michel et al. present a case of a 53-year-old woman with a diagnosis of an AML and NPM1 with PDGFRβ mutations. The patient initially received induction chemotherapy and one cycle of consolidation, followed by allo-SCT on account of persistence of NPM1-positive measurable residual disease (MRD). The patient relapsed three months later; at that point, PDGFRβ rearrangements were given as the reason for recurrence. As she demonstrated no response to intensive reinduction chemotherapy, 400 mg/day imatinib was administered. After achieving a fast hematologic response, a second allo-HCT was performed, and the dosage of imatinib was decreased to 100 mg/day, three times a week. The imatinib maintenance was stopped after 10 months when patient achieved NPM1-negative molecular remission. This led to a molecular relapse in less than a year and a half. Treatment with imatinib was restarted and again, molecular remission was achieved. This example confirms the efficacy of TKI treatmentin AML with PDGFRβ rearrangement [24]. Arefi et al. [13] examined the incidence of myeloproliferative neoplasms (MPN) with PDGFRβ rearrangements and the frequency of responses to imatinib therapy. The findings indicate the effectiveness of imatinib treatment in patients with PDGFRβ mutations. Hence, this kind of rearrangement leads to the production of constitutive enzymatic activity (tyrosine kinase activity) and deregulation of haematopoiesis [13]. The published data generally recommends the use TKI-based treatment in all patients with PDGFRβ rearrangements.
Chan et al. studied 26 cases of patients with MPN and PDGFRβ rearrangements who received therapy with imatinib. Three patients started the imatinib treatment with a dose of 100 mg/day, and all achieved hematologic response in four months. This article suggests that patients with this kind of mutation may be more sensitive to imatinib than those who are CMLBCR-ABL1 positive: four patients who began therapy with a dosage lower than 400 mg demonstrated rapid and durable complete cytogenetic response or molecular remission, and none ofthe seven patients whose imatinib doses were reduced experienced disease progression. Importantly, the reported durability of responses to tyrosine kinase inhibitors indicates thatallo-SCT does not appear necessary incases of chronic MPN with detected PDGFRβ mutation. Unfortunately, this report does not indicate whether deliberate withdrawal of imatinib may be safe, as only three patients stopped treatment [16]. Other tyrosine kinase inhibitors targeting this genetic abnormality, such as dasatinib, pomatinib, sorafenib, quizartinib, suniotinib or sorafenib could also be active in the treatment of AML with PDGFRβ rearrangement [25].
However, none has been introduced into clinical practice. Based on these previous studies, we decided to use a combination of conventional chemotherapy with TKI. Due to the unfavourable experiences with alloHSCT in literature, and the lack of a matched, sibling donor in our patient, we shifted our decision to maintenance treatment. Fortunately, the treatment strategy appeared to be the right: the patient remains in molecular and cytogenetic CR, with a DFS almost 3.5 years. AlloHSCT could be saved for any potential relapse, if it occurs. However, it remains an open question whether we may interrupt the imatinib course: the patient is in a permanent relationship, and would like to have children.
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloidneoplasms and acute leukemia. Blood 127 (2016): 2391-405.
- Seer Is An Authoritative Source For Cancer Statistics In The United States. National Cancer Institute (2021).
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129 (2017): 424-427.
- Shah A, Andersson TM, Rachet B, et al. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol 162 (2013): 509-16.
- Ejduk A, Majewski M, Warzocha K. Znaczenie prognostyczne aberracji genetycznych w ostrej bialaczce szpikowej. Acta Haematologica Polonica 37 (2006): 5-24.
- Guérit E, Arts F, Dachy G, et al. PDGF receptor mutations in human diseases. Cell Mol Life Sci 78 (2021): 3867-3881.
- Klug LR, Kent JD, Heinrich MC. Structural and clinical consequences of activation loop mutations in class III receptor tyrosine kinases. PharmacolTher 191 (2018): 123-134.
- Rosnet O, Birnbaum D. Hematopoietic receptors of class III receptor-type tyrosine kinases. Crit Rev Oncog 4 (1993): 595-613.
- Nordby Y, Richardsen E, Rakaee M, et al. High expression of PDGFR-β in prostate cancer stroma is independently associated with clinical and biochemical prostate cancer recurrence. Sci Rep 7 (2017): 43378.
- Williams LT. Signal transduction by the platelet-derived growth factor receptor. Science 243 (1989): 1564-1570.
- Farooqi AA, Siddik ZH. Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape. Cell Biochem Funct 33 (2015): 257-265.
- Naymagon L, Marcellino B, Mascarenhas J. Eosinophilia In acute myeloid leukemia: Overlooked and underexamined. Blood Reviews (2019): 23-31.
- Arefi M, García JL, Peñarrubia MJ, et al. Incidence and clinical characteristics of myeloproliferative neoplasms displaying a PDGFRβ rearrangement. Eur J Haematol 89 (2012): 37-41.
- Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J ClinOncol 30 (2012): 2441-2448.
- Pluta A, Robak T, Brzozowski K, et al. Early induction intensification with cladribine, cytarabine, and mitoxantrone (CLAM) in AML patient streated with the DAC induction regimen: a prospective, non-randomized, phase II study of the Polish Adult Leukemia Group (PALG). LeukLymphoma 61 (2020): 588-603.
- Chan CY, Burbury K, Apperley JF, et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 123 (2014): 3574-3577.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114 (2009): 937-951.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127 (2016): 2375-2390.
- Bain BJ. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1. Haematologica 95 (2010): 696-698.
- Andrei M, Bandarchuk A, Abdelmalek C, et al. PDGFR?-Rearranged Myeloid Neoplasm with Marked Eosinophilia in a 37-Year-Old Man; And a Literature Review. Am J Case Rep 18 (2017): 173-180.
- Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol 93 (2018): 1267-1291.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129 (2017): 424-447.
- Chang H, Chuang WY, Sun CF, et al. Concurrent acute myeloid leukemia and T lymphoblastic lymphoma in a patient with rearranged PDGFRB genes. Diagnostic Pathology 7 (2012): 19.
- Michel C, Mack EKM, Mais CN, et al. Cloning and characterization of a novel druggable fusion kinase in acute myeloid leukemia. Haematologica 105 (2020): 395-398.
- Heldin C-H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal 11 (2013): 97.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
