58-Year-Old Female with Persistent Fever and Confusion
Article Information
Jagdish Shukla*, Geetanjali Kumar, Daniel Asher
Clinic Director, Family Practice Center, Associate Program Director, Family Medicine Residency Program, GA, Georgia
*Corresponding Author: Jagdish Shukla, Clinic Director, Family Practice Center, Associate Program Director, Family Medicine Residency Program, Columbus, GA 31901, Georgia
Received: 08 October 2021; Accepted: 22 October 2021; Published: 01 November 2021
Citation: Jagdish Shukla, Geetanjali Kumar, Daniel Asher. 58-Year-Old Female with Persistent Fever and Confusion. Archives of Clinical and Medical Case Reports 5 (2021): 778-791.
View / Download Pdf Share at FacebookKeywords
Chest pain; Emergency medicine; Rapid Plasma
Article Details
1. Background
A 58-year-old African American female presented to the emergency department with chief complaints of subjective low-grade fever and generalized weakness for two weeks associated with difficulty in ambulation which gradually worsened. Patient denied a history of similar symptoms in the past. Family members reported mild confusion associated with subjective fever. Patient denied chest pain, shortness of breath, cough, nausea, vomiting, abdominal pain, dark stools or blood in stools, bowel/bladder incontinence, focal weakness, numbness, dizziness, falls or seizure. Past medical history was significant for benign essential hypertension, gastroesophageal reflux disease, mixed hyperlipidemia, cannabis abuse, history of cerebrovascular accident with residual right upper and lower extremity weakness. Patient had no known drug allergies. Family and social history were unremarkable. Home medications were Atorvastatin, Aspirin and Amlodipine.
2. Physical Examination and Work up
Upon presentation to the emergency room her vitals were Height 6”, weight 205lbs, blood pressure 108/64 mm Hg, pulse 96 beats/min, and respiratory rate 18 breaths/min, temperature is 99.9 degrees Fahrenheit. General physical examination revealed a slightly confused but comfortable-appearing woman in no acute distress. The head and neck examination findings were normal, and no scleral icterus was noted. No nuchal rigidity. She did not appear dehydrated. Systematic physical examination showed clear lung sounds bilaterally. The S1 and S2 heart sounds were normal, with no audible murmurs, rubs, or gallops. There was 2+ pitting edema in bilateral lower extremities. The patient had a soft abdomen, non-distended, with normoactive bowel sounds heard in all four quadrants. Patient was awake, alert, oriented to time, place, and person, GCS-14. Mild confusion was noted on the physical exam. Her answers to some simple questions were incomprehensible which was abnormal for her. Cranial nerves intact. Residual decrease in tone of RUE and RLE from previous CVA, at baseline. Motor strength was 5/5 in the left upper and lower extremity and ? in the right upper and lower extremity which is baseline for her. Sensation to pain, touch, pressure intact in bilateral upper and lower extremities. Deep tendon reflexes normal bilaterally. Patient was subsequently admitted for acute encephalopathy.
Lab work showed normal white cell count, microcytic anemia (no h/o anemia) and normal thyroid hormone test. 2 sets of Blood cultures were negative for any growth, urinalysis was negative for infection and urine drug screen was positive for cannabis. Chest x ray was unremarkable, and electrocardiogram showed normal sinus rhythm. Ammonia levels were within reference range. Brain CT was significant for mild atrophy with focal encephalomalacia in the left basal ganglia with ex- vacuo dilatation of the frontal horn of the left lateral ventricle suggesting sequela of old vascular insult without any acute abnormality. Vitamin B12 levels were normal and syphilis screen with Rapid Plasma Reagin (RPR) test was reactive. Fluorescent treponemal antibody absorption test and lumbar puncture test were ordered along with an Infectious disease consultation.
|
CBC |
Anemia Panel |
||
|
WBC |
9.6 10^3/ul |
Iron |
12 ug/dL |
|
RBC |
3.94 10^6/ul |
TIBC |
210 ug/dL |
|
Hemoglobin |
7.8 g/dL |
Iron Saturation |
6% |
|
Hematocrit |
28.30% |
UIBC |
198.0 ug/dL |
|
MCV |
71.7 fL |
Ferritin |
1,554.0 ng/mL |
|
MCH |
19.7 pg |
Haptoglobin |
653.0 mg/dL |
|
MCHC |
27.5 g/dL |
Lactate Dehydrogenase |
207 U/L |
|
RDW |
17.50% |
Retic Ct % |
2.85% |
|
MPV |
8.2 fL |
TSH |
1.940 ulU/mL |
|
Platelets |
540 10^3/ul |
Vitamin B12 |
429 pg/mL |
|
Basic Metabolic Panel |
Hepatic Function Panel |
||
|
Sodium |
133 mmol/L |
Total Protein |
6.8 g/dL |
|
Potassium |
4.1 mmol/L |
Albumin |
2.0 g/dL |
|
Chloride |
96 mmol/L |
ALT |
28 U/L |
|
CO2 |
24 mmol/L |
AST |
59 U/L |
|
Glucose |
138 mg/dL |
Alkaline Phosphatase |
58 U/L |
|
BUN |
13 mg/dL |
Total Bilirubin |
1.3 mg/dL |
|
Creatinine |
0.65 mg/dL |
Bilirubin, Direct |
1.00 mg/dL |
|
Calcium |
8.2 mg/dL |
Bilirubin, Indirect |
0.3 mg/dL |
|
Anion Gap |
17 |
Albumin/Globulin Ratio |
0.4 |
|
BUN/Creatinine ratio |
20 |
||
|
GFR MDRD Af Amer |
>60 mL/min/1.73sqm |
||
On the night of admission, patient developed a temperature of 102.9 degrees Fahrenheit, became tachycardic with a heart rate between 120-140 bpm, tachypneic with a respiratory rate of 28 bpm, she was started on broad spectrum IV antibiotics IV Vancomycin and Meropenem (PCN allergy). Repeat blood cultures, urinalysis, urine culture, chest x-ray, lactic acid, CRP were ordered. Infectious Disease physician ordered HIV, ANA titer and pattern, rheumatoid factor, anti-citrullinated peptide antibodies and TB skin testing. Acute Hepatitis panel was significant for a reactive Hepatitis C antibody. Hepatitis C Virus (HCV) RNA quantitative test yielded a result of 734,000 IU/ml. HIV was non-reactive. Tuberculosis skin testing was negative. Lumbar Puncture was negative for any infectious etiology.
3. CSF Analysis
|
Glucose |
72 mg/dL |
|
Protein |
26.4 mg/dL |
|
Tube Number |
3 |
|
Color |
Colorless |
|
Clarity |
Clear |
|
WBC |
1 /mm3 |
|
RBC |
0 |
|
Lymph |
52% |
|
Monocytes |
48% |
|
Cells Counted |
100 |
|
VDRL, CSF |
Non-reactive |
3.1 Consultations
- Infectious Disease: Consulted to assist with evaluation of fever of unknown origin as well as management of syphilis infection.
- Gastroenterology: Consulted to rule out GI source of anemia as well as management of hepatitis C.
- Hematology/Oncology: Consulted to assist with evaluation of acute microcytic anemia.
Fluorescent treponemal antibody absorption test was reactive. The Venereal disease research laboratory (VDRL) test for CSF was non-reactive as was the Treponema Pallidum Antibody, IFA for CSF. CSF HSV negative. Patient was started on a 28-day course of PO Doxycycline 100mg twice daily for treatment of late latent syphilis. Computed Tomography of abdomen and pelvis (CT) was performed to evaluate possible sources of severe anemia. No significant results were yielded from the CT of the abdomen and pelvis. Hemoglobin and hematocrit continued to decrease during hospitalization to the point that the patient required a blood transfusion. Fecal occult blood test was negative. Gastroenterologist recommended a colonoscopy which the patient declined, and the gastroenterologist agreed to see the patient as an outpatient for treatment of chronic hepatitis C. Hematologist ordered hemoglobin electrophoresis and reviewed peripheral blood smear. Peripheral blood smear was significant for polychromasia, anisocytosis and platelet clumping. Hemoglobin electrophoresis illustrated normal levels of HbA2 and HbF without any variants. Reticulocyte count was within reference range; however, reticulocyte hemoglobin content (CHr) was decreased at 22.5pg. Autoimmune workup including ANA IFA screen, rheumatoid factor, cyclic citrullinated peptide antibodies, lactate dehydrogenase, C3/C4 complement, cryoglobulin, were within normal reference ranges. The patient continued to be febrile during her second week of hospital stay despite broad spectrum antibiotics and antipyretics and negative repeat urine, blood cultures and negative lumbar puncture.
Question 1.
What is the most likely cause of this patient’s fever?
- Neurosyphilis
- Viral encephalitis
- Bacterial meningitis
- Fever of unknown origin
Answer: D
Despite extensive workup, no etiology was found for her persistent fever. Fever of unknown origin (FUO) in adults is one of the most vexing clinical conditions for clinicians and patients. There are no published guidelines, nor is there a recommended standard approach to the diagnosis. The definition of what constitutes FUO remains controversial. FUO was first described in a 1961 case series as prolonged febrile illness (temperature of 101degree F (38.3 degree Celsius) or higher for three weeks or longer that did not have an established etiology despite a one-week inpatient evaluation. The arbitrary guideline of three weeks allowed most acute, self-limited illnesses to resolve, as well as sufficient time to complete the initial investigation [1].
3.2 Epidemiology
The epidemiology of FUO is broad and depends on the etiology, age group, geography, environmental exposure, and immune/HIV status. In developing countries, infectious etiology of FUO is most prevalent whereas in developed countries FUO is likely due to non-infectious inflammatory disease [2].
3.3 Etiology and differential diagnosis
Causes of fever of unknown origin (FUOs) are diverse and broken into four major subgroups, as summarized below. Failure to reach a definitive diagnosis in patients presenting with FUO is not uncommon; 20 percent of cases remain undiagnosed [3].
- Infectious: Tuberculosis especially extra pulmonary sites, abdominal and pelvic abscesses, subacute bacterial endocarditis, sinusitis, osteomyelitis, and dental abscess, human immunodeficiency virus, syphilis, cytomegalovirus, epstein –barr virus, lyme disease
- Rheumatological/inflammatory: rheumatoid arthritis, rheumatic fever, systemic lupus erythematosus, adult still’s disease, temporal arteritis, polymyalgia rheumatica, inflammatory bowel disease, vasculitis, reiter’s syndrome
- Malignant/neoplastic: chronic leukemia, lymphomas, renal cell carcinoma, metastatic cancers, renal cell carcinoma, myelodysplastic syndrome, sarcomas
- Miscellaneous disorders: drug induced fever, deep venous thrombosis, hepatitis, factitious fever, sarcoidosis
3.4 Evaluation of the patient with FUO
The initial approach to the patient presenting with fever should include a comprehensive history, physical examination, and appropriate laboratory testing. The first step should be to confirm a history of fever and document the fever pattern. History of recent travel, exposure to pets and other animals, the work environment, and recent contact with persons exhibiting similar symptoms. The family history should be carefully scrutinized for hereditary causes of fever, such as familial Mediterranean fever. Finally drug induced fever must be considered in patients who are taking medications. Diagnostic clues often are not readily apparent on physical examination: repeated examination may be essential with careful attention to skin, mucus membrane, and lymphatic system, as well as abdominal palpation for masses or organomegaly, is important.
A cost-effective individualized approach is essential to the evaluation of these patients, and without a thoughtful and focused investigation, inappropriate tests might be performed. The preliminary investigation should include complete blood count, liver function test, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), urinalysis, and basic cultures. A chest radiograph should be obtained in all patients to screen for infection, collagen vascular disease, or malignancy. Skin testing for tuberculosis with purified protein derivative (PPD) or interferon gamma assay should be considered in all patients [3]. Procalcitonin is a newer marker specific for bacterial infection. Elevated lactate dehydrogenase levels can be indicative of infectious and malignant causes of FUO including malaria, lymphoma, and leukemia. Measurement of ferritin levels may also be helpful. An elevated ferritin
level in the prolonged febrile illness may indicate malignancy (especially myeloproliferative disorders). Testing for antinuclear antibodies, rheumatoid factor, human immunodeficiency virus, Epstein-Barr virus, cytomegalovirus, anti-neutrophilic cytoplasmic antibodies, as well as measurement of the creatine kinase level, can suggest other infectious sources and common noninfectious inflammatory disease etiologies, such as systemic lupus erythematosus, vasculitis [1]. Other recommended blood tests include cryoglobulin (elevated in systemic lupus erythematosus, leukemias and lymphomas), complement studies, serological test, peripheral smear, serum electrophoresis, and thyroid function studies [1].
3.5 Imaging studies
If this initial assessment does not disclose the source of fever, more specific investigatory techniques, such as serology, sonography, computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine scanning should be conducted, based on clinical suspicion early in the diagnosis process to rule out such common causes of FUO as intra- abdominal abscess or malignancy. (3) Doppler ultrasonography is indicated for suspected thromboembolism. Magnetic resonance imaging of the aortic arch and great vessels of the neck was shown to be helpful when vasculitis was suspected. Nuclear imaging studies are noninvasive, image the whole body, and can localize a potential infectious or inflammatory cause for FUO. Recently 18F fluorodeoxyglucose positron emission tomography technology has been evaluated for guiding further invasive testing, especially in patients who have elevated ESR or CRP levels. The 18F Fluorodeoxyglucose is taken up by the inflammatory and cancer cells because of their high rate of glycolysis. Several studies examining this method in patients with FUO found diagnostic yields running from 16% to 69%, with a high positive predictive value (93%) and negative predictive value (100%) [1].
3.6 Biopsies
More invasive testing, such as lumbar puncture or biopsy of bone marrow, liver, or lymph nodes, should be performed only when clinical suspicion shows that these tests are indicated or when the source of the fever remains unidentified after extensive evaluation.
3.7 Referral
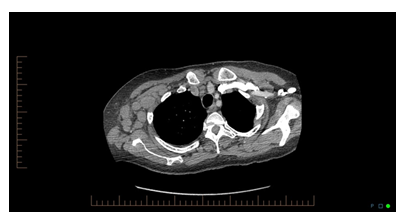
When the definitive diagnosis remains elusive and the complexity of the case increases, an infectious disease, rheumatology, hematologist/oncology consultation may be helpful [3]. On the tenth day of hospitalization, patient developed a new onset cough, shortness of breath, and rhonchi upon a physical exam. Chest x ray was unremarkable. Chest CT was ordered for further evaluation and was significant for small right-sided pleural effusion with associated compressive atelectasis/infiltrate, mild subcarinal adenopathy with prominence of the middle mediastinal and right hilar lymph nodes, mild to moderate anterior pericardial adenopathy, and very large right axillary and subpectoral adenopathy.
Question 2
CT chest image is shown below, looking at the following CT images, what is the most probable diagnosis?
- Sarcoidosis
- Lymphoma
- Interstitial Lung disease
- Lung abscess
Answer: B
Chest CT was significant for large right axillary lymphadenopathy, and the treating physician’s team agreed to do a biopsy of the right axillary lymph node. General surgery was consulted for excisional lymph node biopsy. Surgical pathology exam was significant for diffuse large B cell lymphoma, activated B cell type. Flow cytometry was significant for B cell lymphoma with increased side and forward scatter, and negative for CD5 and CD10 antigens. Lymphoma represents group of malignant neoplasms of lymphocytes, which can involve lymphatic tissue, bone marrow, or extra nodal sites. Thomas Hodgkin published the first description of lymphoma in 1832 [4]. Since then, many other forms of lymphoma have been described. It traditionally classified as Hodgkin lymphoma (HL) and Non-Hodgkin lymphoma (NHL). The World Health Organization’s classification system identifies more than 90 different subtypes (Table 1) [5-7]. NHL are cancers of mature B, T, and Natural Killer (NK) cells and can be classified as either a mature B-NHL, or a mature T/NK-NHL. About 90% of all lymphomas are of B-cell origin [8]. This article will focus on Diffuse large B cell Lymphoma (DLBCL).
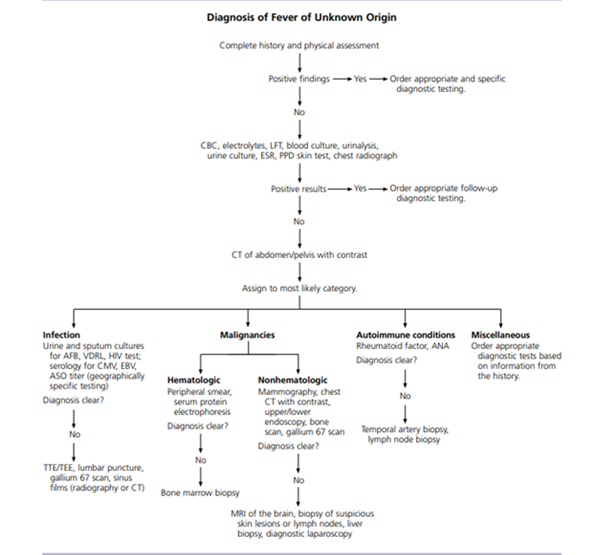
Figure 1: Algorithm for the diagnosis of fever of unknown origin. (Reproduced with permission from Approach to the Adult Patient with Fever of Unknown Origin, December 1,2003, Vol 68, No 11, issue of American Family Physician Copyright @ 2003 American Academy of Family Physicians, ALL Rights Reserved.
|
Lymphoma Subtype |
Incidence per 100,000 |
Five Year Survival |
|
Hodgkin |
2.8 |
85.7% |
|
Non-Hodgkin B-Cell Lymphoma |
||
|
Burkitt |
0.4 |
64.1% |
|
Diffuse Large B Cell |
7.2 |
63.2% |
|
Follicular |
3.5 |
88.4% |
|
Marginal Zone |
2.2 |
90.3% |
|
Precursor B Cell |
1.5 |
68.9% |
|
Non-Hodgkin T-Cell and Natural Killer Cell Lymphoma |
||
|
Mycosis Fungoides |
0.6 |
90.9% |
|
Peripheral T-cell |
1.2 |
58.4% |
Reproduce with permission from Lymphoma: Diagnosis and Treatment; Jan 1,2020, Vol 101, No 1, issue of American Family Physician Copyright@2020 American Academy Family Physicians.
Table 1: Common Lymphoma subtypes with Incidence and Five Year Survival.
4. Epidemiology
About 82,000 new patients are projected to be diagnosed with lymphoma in 2019, representing 4.7% of all new cancer cases and 55.6% of all blood cancers in the United States. The current five-year survival rate for NHL is 72.0%. Almost 21,000 people are projected to die from lymphoma in 2019, representing 3.5% of all cancer deaths. Incidence of NHL is higher in men and whites, and it increases with age. The median age of patients at diagnosis of non-Hodgkin lymphoma is 67 years, and the median age at death is 76 [9, 10]. Patients with both inherited or acquired immunodeficiency states are predisposed to developing NHL [8].
5. Risk Factors
Environmental, genetic, infectious, and inflammatory etiologies increase the risk of lymphoma. First-degree relatives of patients with NHL have 1.7-fold increase in incidence. There are three main mechanisms through which infection increases lymphoma risk: direct transformation of lymphocytes (Epstein-Barr virus causing Burkitt lymphoma, HL and NHL), immunosuppression (HIV causing HL and NHL), and chronic antigenic stimulation (Helicobacter pylori, Chlamydia psittaci, Campylobacter jejuni, Campylobacter coli, Borrelia burgdorferi causing NHL/Mucosa associated lymphoid tissue) [11, 12]. Modifiable risk factors include current or former tobacco use and obesity (BMI>30 or higher). Breast implants and long-term pesticide exposure have also been associated with NHL [13-16]. Rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, dermatomyositis, and celiac disease are inflammatory conditions that increase the risk of lymphoma through disease-specific causes and the chronic use of immunosuppressive medications [17].
6. Clinical Presentation
Lymphoma commonly presents as painless adenopathy which can wax and wane over years or involve rapidly progressive adenopathy in more aggressive subtypes. NHL can originate anywhere in the body, with specific subtypes originating in the gastrointestinal tract, skin, or central nervous system. Systemic symptoms of fever, unexplained weight loss, and night sweats occur in a subset of patients with more advanced disease. Lymphoma spreads to extra-nodal sites by direct invasion or by hematogenous spread to the spleen, liver, lungs, or bone marrow [18, 19]. High-grade lymphomas can present as oncologic emergencies because of the structural compression from the enlarging tumor, including superior vena cava syndrome, malignant epidural spinal cord compression, or malignant pericardial effusion [20].
Generally, lymph node > 2cm, which lasted for more than 4-6 weeks and progressively increasing in size in the absence of any other obvious condition that explains lymphadenopathy requires biopsy [21-23]. DLBCL is the most common histologic form of NHL and represents one third of all cases. Majority of the patients with DLBCL presents with advanced stage disease, 40% will have B symptoms, 50% will have elevated LDH levels, about 40% will have involved of extra nodal sites. The tumor consists of a diffuse proliferation of large, atypical lymphocytes with a high proliferative index. These cells typically express the B-cell antigens CD19, CD20, and CD79a [8].
7. Diagnosis
The diagnosis of lymphoma is made using an open lymph node biopsy, based off morphology, immunohistochemistry, and flow cytometry [24]. In the absence of an obvious site to biopsy, bone marrow aspiration/biopsy may provide critical diagnostic information, as may the use of computed tomography (CT) or positron emission tomography (PET) scanning to detect sites of disease suitable for biopsy [25]. Fresh tissue, and in some cases fixed tissue, can be studied for cytogenetic abnormalities, chromosomal translocations, immunoglobulin or T cell receptor (TCR) gene rearrangements, gene expression analysis, and DNA sequencing [25].
8. Staging
The Ann Arbor staging system was initially developed in 1971. The Lugano classification (Table 2) system further modified staging by incorporating positron emission tomography/computed tomography (PET-CT) results to determine the staging of the lymphoma [26].
|
Stage* |
Description of Disease from PET / CT Results |
|
I |
Single nodal group or Single extra lymphatic lesion |
|
II† |
Multiple nodal groups on same side of diaphragm or with limited contiguous extra lymphatic involvement |
|
III |
Multiple nodal groups on both sides of the diaphragm; may involve spleen |
|
IV |
Noncontiguous extra lymphatic involvement |
*—Staging for Hodgkin lymphoma is further subdivided for systemic symptoms; A for absence of symptoms or B for fevers > 101.3°F (38.5°C), drenching night sweats, or 10% (of body weight) unintentional weight loss over the past six months.
†—Stage II may also be classified as bulky disease (> 10-cm mass), which may be treated as limited or advanced disease based on several prognostic factors.
Table 2: Lugano Classification for Staging Lymphoma.
9. Prognosis
The International Prognostic Index is used broadly for all subtypes of NHL (Table 3). 5-year overall survival rate with anthracycline based regimen is as follows:
Score 0 to 1=73%, score 2=51% score 3=43%, score 4 to 5=26% [27].
3-year overall Survival rate improved when CD20 positive aggressive lymphoma patients received R-CHOP or CHOP like chemotherapy as follows:
0 to 1=91%, 2=81%, 3=65%, 4 to 5=59 [8].
9.1 Treatment
Treatment of lymphoma consists of chemotherapy alone or in combination with radiotherapy. Radiotherapy alone is not recommended [28]. Treatment for non-Hodgkin lymphoma varies depending on the histology, but often uses treatments such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone; with short term complication like constipation, neuropathy, hyperglycemia and heart failure and long-term complications like cardiomyopathy, myelosuppression), with or without rituximab (Rituxan; R-CHOP), a monoclonal antibody specific for CD20-positive B lymphocytes [29].
|
Age > 60 |
1 |
|
Elevated Serum Lactate Dehydrogenase |
1 |
|
ECOG performance status 2 |
1 |
|
Ann Arbor stage III or IV disease |
1 |
|
Extra nodal site involvement > 1 |
1 |
ECOG(Eastern Cooperative Oncology Group) performance status: 0= fully active, 1= ambulatory but restricted to light work, 2= ambulatory but unable to carry out activities, 3= limited self-care only, 4= bedridden, 5= dead [27].
Table 3: International Prognostic Index.
10. Interim Reassessment
PET-CT scans, and subsequent Deauville scoring (Table 4) [30], should be used to assess the response to chemotherapy in Lymphoma [28, 31]. A score of 3 or less is considered complete remission in non-Hodgkin lymphoma and should conclude the current treatment course. A score of 4 or 5 is an indicator to consider escalating therapy. Score of 3-4 should consider additional chemo and or radiotherapy, while score of 5 indicates needs for biopsy and positive biopsy indicates refractory disease [28].
|
PET / CT Finding |
Score |
|
No FDG uptake related to lymphoma |
1 |
|
FDG uptake at lymphoma site is < mediastinum FDG uptake |
2 |
|
FDG uptake at lymphoma site is > mediastinum FDG uptake but < liver FDG uptake |
3 |
|
FDG uptake at lymphoma site is > liver FDG uptake at any site |
4 |
|
FDG uptake at lymphoma site is substantially > liver FDG uptake or new FDG uptake sites found |
5 |
FDG = fluorodeoxyglucose; PET-CT = positron emission tomography/computed tomography.
Table 4: Deauville Score for Assessing PET-CT Scans.
11. Relapse
The most common subtype, diffuse large B-cell lymphoma, has a 40% lifetime relapse rate [32]. These patients may still be cured with salvage chemotherapy regimens followed by autologous stem cell transplantation. Patients with a poor performance status or advanced age that are not candidates for such an approach, however, are often managed with palliative intentions. Radiation to symptomatic areas of disease can be transiently helpful [8].
12. Surveillance & Immunizations
Patients who have achieved remission need routine surveillance to monitor for complications and relapse [33]. Current National Comprehensive Cancer Network (NCCN) guidelines outline specific monitoring parameters for follow-up and prevention of secondary disease [28, 34-37].
12.1 Cancer screening
Breast cancer screening by annual mammography should be started at the age 40. If patient has chest or axilla radiation; start screening mammogram 8 to 10 years after treatment or at age 40 whichever comes first. Consider Breast MRI (Magnetic Resonance Imaging) if chest radiation was received between ages 10 and 30. Perform routine surveillance tests for cervical, colorectal, lung and prostate cancers per USPSTF (United State Preventive Service Task Force) guidelines [33].
12.2 Laboratory screening
Patient should get complete blood count, fasting blood glucose, and comprehensive metabolic panel annually, Lipid profile per USPSTF guideline, Thyroid- stimulating hormone if patient had neck irradiation.
12.3 Cardiac screening
Annual blood pressure screening, lifestyle modification, and treatment of obesity, hypertension, and tobacco use. Consider stress test and or echocardiography at 10-year intervals based on other associated risk factors. Carotid ultrasonography every 10 years if patient had neck irradiation.
12.4 Counseling
Annual depression screening should be performed. Neurocognitive impairment screening should be performed for any patient who is at high risk due to intrathecal treatment or brain irradiation. Consider referral to reproductive endocrinology if patient is facing issues with infertility.
12.5 Immunization
Age appropriate immunizations per Center for Disease Control (CDC) including annual influenza vaccine; resume live vaccine at least 3 months after completion of chemotherapy. PCV13 (13-valent pneumococcal conjugate vaccine) followed by PPSV23 (23-valent pneumococcal polysaccharide vaccine) at least 8 weeks later and again five years later. Patient who had hematopoietic stem cell transplantation should receive three doses Herophilus Influenzae type b(Hib). If a patient is asymptomatic, routine surveillance imaging does not improve outcomes or provide a clinical benefit [34, 35]. NCCN imaging guidelines for lymphoma surveillance state that it is acceptable to perform chest radiography or CT of the chest every six to 12 months for the first two years and then yearly for the next three to five years posttreatment [35]. Surveillance imaging with PET-CT scans following complete remission is not recommended [34, 35]. Disease marker research is ongoing, examining minimal residual disease measurements, a polymerase chain reaction–based method that looks at identifying tumor-specific DNA sequences [35].
Question: 3
Following statements are True regarding Lymphoma EXCEPT:
- Generally, lymph node > 1cm, which lasted for more than 4 weeks and progressively increasing in size in the absence of any other obvious condition that explains lymphadenopathy requires biopsy
- The diagnosis of lymphoma is made using an open lymph node biopsy, based off morphology, immunohistochemistry, and flow cytometry.
- Treatment of lymphoma consists of chemotherapy alone or in combination with radiotherapy.
- In the absence of an obvious site to biopsy, bone marrow aspiration/biopsy may provide critical diagnostic information
Answer: A
13. Clinical Course of Patient
Hematologists decided to start urgent chemotherapy with Rituxan, Cyclophosphamide, Adriamycin, and Vincristine regimen. Persistent fever resolved after the first round of chemotherapy for lymphoma.
References
- Hersch EC, Oh RC. Prolonged febrile illness and fever of unknown origin in adults. Am Fam Physician (2014).
- Cunha BA, Lortholary O, Cunha CB. Fever of unknown origin: A clinical approach. Am J Med (2015).
- Roth A, BAsello G. Approach to the Adult Patient with Fever of Unknown Origin - December 1, 2003 - American Family Physician. Am Fam Physician (2003).
- Hellman S. Thomas Hodgkin and Hodgkin’s Disease. JAMA (1991).
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood (2016).
- National Cancer Institute. Cancer Stat Facts. Surveillance, Epidemiol End Results Progr (2019).
- Lewis WD, Lilly S, Jones KL. Lymphoma: Diagnosis and treatment. Am Fam Physician (2020).
- Jameson JL, Fauci AS, Kasper DL, et al. Editors. In: Harrison’s Principles of Internal Medicine, 20e (2018).
- National Cancer Institute. Non-Hodgkin Lymphoma - Cancer Stat Facts. SEER Stat Fact Sheetsn (2021).
- National Cancer Institute. Cancer Stat Facts: Hodgkin Lymphoma. Cancer Statistics (2021).
- Suarez F, Lecuit M. Infection-associated non-Hodgkin lymphomas. Clin Microbiol Infect (2015).
- Coghill AE, Hildesheim A. Epstein-Barr virus antibodies and the risk of associated malignancies: Review of the literature. Am J Epidemiol (2014).
- Sergentanis TN, Kanavidis P, Michelakos T, et al. Cigarette smoking and risk of lymphoma in adults: A comprehensive meta-analysis on Hodgkin and non-Hodgkin disease. Eur J Cancer Prev (2013).
- Lichtman MA. Obesity and the Risk for a Hematological Malignancy: Leukemia, Lymphoma, or Myeloma. Oncologist (2010).
- Gidengil CA, Predmore Z, Mattke S, et al. Breast implant-associated anaplastic large cell lymphoma: A systematic review. Plast Reconstr Surg (2015).
- Administration UF and D. Questions and answeres about breast implant associated anaplastic large cell lymphoma (2019).
- Yadlapati S, Efthimiou P. Autoimmune/Inflammatory Arthritis Associated Lymphomas: Who Is at Risk?. Biomed Res Int (2016).
- Ansell SM. Non-Hodgkin Lymphoma: Diagnosis and Treatment. In: Mayo Clinic Proceedings (2015).
- Ansell SM. Hodgkin lymphoma: Diagnosis and treatment. In: Mayo Clinic Proceedings (2015).
- Higdon ML, Atkinson CJ, Lawrence K V. Oncologic emergencies: Recognition and initial management. Am Fam Physician (2018).
- Slap GB, Connor JL, Wigton RS, et al. Validation of a Model to Identify Young Patients for Lymph Node Biopsy. JAMA J Am Med Assoc (1986).
- Slap GB, Brooks JSJ, Schwartz JS. When to Perform Biopsies of Enlarged Peripheral Lymph Nodes in Young Patients. JAMA J Am Med Assoc (1984).
- Pangalis GA, Vassilakopoulos TP, Boussiotis VA, et al. Clinical approach to lymphadenopathy. Semin Oncol (1993).
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood (2011).
- Zeppa P, Marino G, Troncone G, et al. Fine-Needle Cytology and Flow Cytometry Immunophenotyping and Subclassification of Non-Hodgkin Lymphoma: A Critical Review of 307 Cases with Technical Suggestions. Cancer (2004).
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. J Clin Oncol (2014).
- A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. N Engl J Med (1993).
- Hoppe RT, Advani RH, Ai WZ, et al. NCCN Guidelines Insights: Hodgkin Lymphoma, Version 1.2018. J Natl Compr Cancer Netw (2018).
- Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol (2006).
- Armitage JO, Gascoyne RD, Lunning MA, et al. Non-Hodgkin lymphoma. Lancet (2017).
- Van Heertum RL, Scarimbolo R, Wolodzko JG, et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: An operational approach for clinical trials. Drug Des Devel Ther (2017).
- Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol 31 (2018): 209-216.
- Force USPST. Published Recommendations (2021).
- Hiniker SM, Hoppe RT. Post-treatment surveillance imaging in lymphoma. Semin Oncol (2017).
- Cohen JB, Kurtz DM, Staton AD, et al. Next-generation surveillance strategies for patients with lymphoma. Futur Oncol (2015).
- El-Galaly TC, Jakobsen LH, Hutchings M, et al. Routine imaging for diffuse large b-cell lymphoma in first complete remission does not improve post-treatment survival: A danishâ€"swedish population-based study. J Clin Oncol (2015).
- Centres for disease control and prevention. General Best Practice Guidelines for Immunization (2019).


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
