COVID-19 and Its Association with New-Onset Diabetes
Article Information
Kunal M. Ajmera MD, MPH*
Department of Hospital Medicine, Calvert Health Medical Center, Prince Frederick, MD, USA
*Corresponding Author: Kunal M. Ajmera, MD MPH, Department of Hospital Medicine, Calvert Health Medical Center, Prince Frederick, MD, USA
Received: 25 October 2021; Accepted: 11 November 2021; Published: 17 November 2021
Citation: Kunal M. Ajmera. COVID-19 and Its Association with New-Onset Diabetes. Archives of Clinical and Medical Case Reports 5 (2021): 855-861.
View / Download Pdf Share at FacebookAbstract
Since the beginning of 2020, people’s lives have been changed worldwide as a result of the ongoing coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Research shows a bidirectional association between COVID- 19 infection and diabetes mellitus. Whether COVID-19 causes new-onset diabetes in those at or without risk is unclear. More data are needed to explore the possible role of SARS-CoV-2 in new-onset diabetes post-infection. Here, the case of a young otherwise healthy patient is described; the patient presented with signs and symptoms of new-onset diabetes requiring insulin and was diagnosed with COVID- 19 at the same time.
Keywords
COVID-19; Diabetes; Pathophysiology; Autoimmunity
Article Details
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2), the novel coronavirus that causes coronavirus disease (COVID-19), was first reported in Wuhan, China, in December 2019, and since then has spread worldwide. The ongoing pandemic has, for over 2 years, resulted in an overburdened healthcare system, both directly, owing to testing and hospitalizations related to COVID-19, and indirectly, from COVID-19 associated comorbidities, such as those experienced by COVID-19 long haulers, or long
COVID-19 [1], and crises resulting from a shortage of personal protective equipment [2].
Since the pandemic’s beginning, more than 700,000 people have died in the USA as of October 20, 2021, according to the Centers for Disease Control (CDC). Of those, at least 15.7% of patients had associated diabetes [3]. According to the CDC, in the USA, at least 15.9% of the population (diagnosed or undiagnosed) has diabetes. Office visits related to diabetes number around 27 million per annum, and 12.8% of emergency room (ER) visits are related to diabetes [4]. There is a bidirectional association between COVID-19 and diabetes mellitus. According to the American Diabetes Association, people with diabetes are at higher risk of developing complications and becoming hospitalized from COVID-19 infection. However, the outcome also depends on the age of the patient, the type of diabetes, and how well diabetes has been managed [5]. Moreover, published case reports related to new-onset diabetes mellitus after COVID- 19 infection have been published [6-8]. Here, we present a case of a relatively young, otherwise healthy patient who presented with symptoms of new-onset diabetes and was diagnosed with COVID-19 at the same time. This report associates COVID-19 infection as a possible etiologic factor for new-onset diabetes.
2. Methods
A systematic review of medical literature with the keywords "COVID-19," "Diabetes, "pathophysiology," "autoimmunity" was performed on PubMed, Google Scholar, and ScienceDirect databases. Articles that included definition, pathophysiology, mechanism of action, and etiology were identified. All articles, including reviews (narrative and systematic), meta-analysis, literature review, randomized controlled trials (RCTs), case-control cohorts,
case series, and case reports, were screened for relevant content. Out of the many articles, full texts of the relevant retrieved articles were accessed and used in this paper, in addition to data obtained from the CDC website. Pertinent information was summarized and organized for ease of understanding.
3. Case Presentation
Our patient was a 56-year-old White woman who had a history of long-standing chronic back pain, for which she was taking oxycodone and gabapentin at home, and who had not been vaccinated for COVID-19. She presented at the ER with the following symptoms: 2 months of increased thirst, increased urinary frequency and urgency, loss of appetite, intermittent blurry vision, intermittent generalized abdominal pain, and a weight loss of 40 lb. The patient decided to come to the hospital after having experienced tingling and numbness in her left hand and fingers in the morning. The patient denied having any associated nausea or vomiting, diarrhea, cough, sore throat, shortness of breath, or chest pain. The patient also denied exposure to any known or suspected case of COVID-19. The patient had a body mass index (BMI) of 29 kg/m2, and denied any current or previous history of smoking cigarettes, drinking alcohol, or abusing drugs. She had previously had a cholecystectomy. The patient’s family history was significant for type II diabetes, through her father. Vital signs measured on arrival showed a temperature of 100.9°F, a heart rate of 96-97 beats per minute, respiratory rate of 15-18 breaths per minute, blood pressure of 112 mmHg/74 mmHg, and oxygen saturation of 96% in room air.
Results of blood tests on the day of admission (day 0) are given in Table 1. Neither a CT scan of the head nor an MRI scan of the brain showed any acute intracranial abnormality.
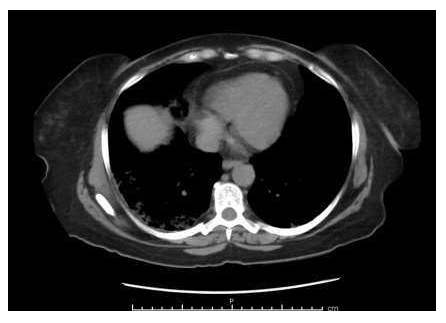
A chest X-ray (Figure 1) showed no acute abnormalities either. A CT scan of the abdomen (Figure 2) showed patchy ground-glass parenchymal opacities bilaterally consistent with COVID-19. There were no acute intra-abdominal findings. Subsequently, the patient tested positive for COVID-19. The patient was started on a course of sliding scale insulin, oral dexamethasone, and supplemental oxygen secondary to hypoxia on ambulation with oxygen saturation of 87%-89% on room air. The tingling sensation and numbness in the patient’s left hand and fingers were thought to be secondary to hyperglycemia.
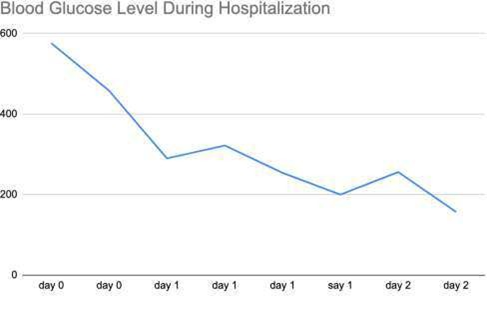
On day 1, the patient’s hemoglobin A1C levels were found to be elevated at >14.0% and the patient was started on a course of oral antihyperglycemic agents, along with long- acting basal insulin. Her blood glucose levels started to improve, from somewhere in the range of 550 mg/dL to below 250 mg/dL (Figure 3).
On day 3, the patient was discharged home in a stable condition and was prescribed 3-4 L oxygen, metformin, glyburide, and Lantus with instructions to attend follow-up sessions closely with the primary care physician. Diabetes education was provided before discharge.
|
Reference Range |
||
|
WBC |
6.0 |
4.0-11.0 10x3/ul |
|
Hb |
12.8 |
13.0-18.0 g/dL |
|
MCV |
87.1 |
80-95 fl |
|
Platelet |
131 |
150-450 10x3/uL |
|
Na |
129 |
136-145 mmol/L |
|
K |
4.3 |
3.5-5.1 mmol/L |
|
Glucose |
576 |
75-100 mg/dL |
|
AG |
9 |
8-16 mEQ/L |
|
S. Creat |
1.3 |
0.6-1.3 mg/dL |
|
Ca |
8.9 |
8.5-10.1 mg/dL |
|
Mg |
1.7 |
1.8-2.4 mg/dL |
|
T. Bili |
0.9 |
0.2-1.0 mg/dL |
|
AST |
108 |
15-37 U/L |
|
ALT |
53 |
12-78 U/L |
|
Alk Phos |
130 |
45-117 U/L |
|
Albumin |
3.4 |
3.4-5.0 g/dL |
|
Lipase |
38 |
73-393 U/L |
Table 1: Blood work on day 0: WBC: White Blood Cell, Hb: Hemoglobin, MCV: Mean Corpuscular Volume, Na: Sodium, K: Potassium, AG: Anion Gap, S. creat: Serum Creatinine, Ca: calcium, Mg: magnesium, T. Bili: Total Bilirubin, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, Alk Phos: Alkaline Phosphatase.
4. Discussion
Since the beginning of the COVID-19 pandemic in early 2020, people’s lives have been changed worldwide. Many people have been impacted directly, owing to COVID-19, whereas others have suffered from pandemic-related disruption in the supply chain. In addition, research shows
that patients with diabetes have higher morbidity and mortality from COVID-19 infection [9-12]. The proposed mechanism of action includes cellular entry of SARS-COV-
2 by binding angiotensin-converting enzyme 2 (ACE2) receptors, causing direct damage and a cytokine storm with the help of T helper cells [13-16]. These receptors are present in the lungs, upper respiratory tract, kidneys, pancreas,
adipose tissue, and small intestine [17-19]. Since ACE2 receptors are overexpressed [20], and interleukin 6 (IL-6) levels are increased [8], in people with diabetes, severe forms of infections are often seen.
However, not much information is available on whether COVID-19 causes new-onset diabetes. There have been reports of cases of diabetes mellitus after COVID-19 infection that were positive for glutamic acid decarboxylase (GAD)-65 antibody (an autoantibody against pancreas) (6) but also reports of similar cases that were negative [7] for glutamic acid decarboxylase (GAD)-65 antibody. A patient with obesity who developed autoimmune type I diabetes after COVID-19-infection, presented by Merchand et al. [6], was found to be positive for GAD-65. In a case series presented by Madam et al. [7], of eight patients with no prior history of diabetes who developed new-onset diabetes mellitus after COVID-19 infection, five out of eight patients tested negative for GAD-65 and required long-term antidiabetic treatment, whereas the remaining three patients were lost to follow-up (personal communication). The mechanism suggested was a direct attachment of COVID-19 spike protein to ACE receptors, resulting in damage to pancreatic islet cells [21] and downregulation of ACE-2 and ACE-2 receptor expression, leading to unopposed angiotensin II, ultimately causing impaired insulin secretion [6-8]. This hypothesis and the role of autoimmunity would need to be explored in a broader clinical trial, as it is not entirely clear whether COVID-19 changes glucose metabolism or causes diabetes through a completely novel mechanism of action. It is also yet to be understood why some patients test positive for autoantibodies against the pancreas and others develop diabetes without the presence of autoantibodies. Early detection of diabetes in COVID-19 patients can also help identify the disease in its early stages
and prevent more severe metabolic complications, such as diabetic ketoacidosis. The patient presented here had a family history of diabetes, a known risk factor for developing diabetes. It is possible that our patient had already contracted COVID-19, but asymptomatically, and that the infection might have pushed her off the cliff, as it were, resulting in the development of full-blown diabetes.
5. Conclusion
With the ongoing COVID-19 pandemic, new data on vaccines, disease complications, and treatment are emerging almost daily. Further, patients with COVID-19 who also have known risk factors, such as a family history of diabetes mellitus or obesity, should be carefully monitored for hyperglycemia and the development of diabetes after recovery.
Disclosure
The authors report no conflicts of interest (financial or non- financial) in this work.
Funding
The author has not declared receiving any grant/funding for this research from any funding agency in the public, commercial or not-for-profit sectors.
References
- Jason LA, Islam M, Conroy K, et COVID-19 Symptoms Over Time: Comparing Long-Haulers to ME/CFS. Fatigue 9 (2021): 59-68.
- Livingston E, Desai A, Berkwits M. Sourcing Personal Protective Equipment During the COVID-19 JAMA. 323 (2020): 1912-1914.
- COVID-19 Mortality Provisional DeathCounts for Coronavirus Disease 2019 (COVID-19).
- Diabetes, Center for Disease Control and pervention, Last accessed 10/20/2021
- How COVID-19 Impacts People with Diabetes, COVID-19 And American Diabetes Association (ADA).
- Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol 57 (2020): 1265-1266.
- Madam N, Katkar Case Series: COVID-19 Infection Causing New-Onset Diabetes Mellitus? Endocr Pract. 27 (2021): S26.
- Chee YJ, Ng SJH, Yeoh Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract 164 (2020): 108166.
- Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev (2020):
- Singh AK, Gupta R, Ghosh A, et Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14 (2020): 303-310.
- Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid- 19 in Critically Ill Patients in the Seattle Region - Case N Engl J Med 382 (2020): 2012-2022.
- Mantovani A, Byrne CD, Zheng MH, et Diabetes as a risk factor for greater COVID-19 severity and in- hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 30 (2020): 1236- 1248.
- Ajmera KM, Wilkinson Heather. Spontaneous Lower Gastrointestinal Bleeding Following Casirivimab/Imdevimab Treatment for COVID-19 Infection: A Case Presentation and Short Literature Archives of Clinical and Biomedical Research 5 (2021): 756-762.
- Gandhi RT, Lynch JB, Del Rio C. Mild or Moderate Covid-19. N Engl J Med 383 (2020): 1757-1766.
- Aleem A, Slenker AK. Monoclonal Antibody Therapy for High-Risk Coronavirus (COVID 19) Patients with Mild to Moderate Disease In: Stat Pearls. Treasure Island (FL): Stat Pearls Publishing (2021).
- Stratton CW, Tang YW, Lu H. Pathogenesis-directed therapy of 2019 novel coronavirus J Med Virol 93 (2021): 1320-1342.
- Vaduganathan M, Vardeny O, Michel T, et al. Renin- Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med 382 (2020): 1653-1659.
- Ajmera Fatal Case of Rhabdomyolysis Post- COVID-19 Vaccine. Infect Drug Resist. 14 (2021): 3929-3935.
- Hoffmann M, Kleine-Weber H, Krüger N, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS- coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target BioRxiv (2020).
- Roca-Ho H, Riera M, Palau V, et al. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Int J Mol Sci 18 (2017): 563.
- Yang JK, Lin SS, Ji XJ, et Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47 (2010): 193-199.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
