Successful Pregnancy Outcome in a Patient with Pompe Disease Receiving Enzyme Replacement Therapy: A Case Report and Review of the Literature
Article Information
Sheela Nampoothiri1*, Dhanya Yesodharan1, Shwetha Kuthiroly1, Nitu Puthenveettil2, Vinitha Murali3, Priyanka Peethambaran3, Radhamany K3, Priya S Kishnani4
1Department of Pediatric Genetics, Amrita Institute of Medical Sciences and Research Center, Cochin, Kerala, India
2Department of Anaesthesia and Critical Care, Amrita Institute of Medical Sciences and Research Center, Cochin, Kerala, India
3Department of Obstetrics and Gynaecology, Amrita Institute of Medical Sciences and Research Center, Cochin, Kerala, India
4Division of Medical Genetics, Duke University Medical Center, Durham, USA
*Corresponding Author: Sheela Nampoothiri, Department of Pediatric Genetics, Amrita Institute of Medical Sciences and Research Center, Cochin, Kerala, India
Received: 01 January 2022; Accepted: 14 February 2022; Published: 28 February 2022
Citation:
Sheela Nampoothiri, Dhanya Yesodharan, Shwetha Kuthiroly,Nitu Puthenveettil, Vinitha Murali, Priyanka Peethambaran, Radhamany K, Priya S Kishnan. Successful Pregnancy Outcome in a Patient with Pompe Disease Receiving Enzyme Replacement Therapy: A Case Report and Review of the Literature. Archives of Clinical and Medical Case Reports 6 (2022): 137-148.
View / Download Pdf Share at FacebookAbstract
Pregnancy in a woman with Pompe disease should be considered to be high risk and requires close monitoring as pregnancy may worsen symptoms, or cause initial symptoms to progress. Clinical data regarding the use of Enzyme Replacement Therapy (ERT) during pregnancy in Pompe disease is available only for 13 patients and the continuation of ERT during lactation has been reported in only one case. We report a successful pregnancy outcome in a 21 year old primipara with juvenile Pompe disease who continued receiving ERT throughout the pregnancy and lactation. She delivered a healthy full term female infant following caesarean section. She experienced worsening of motor function in the third trimester of pregnancy, but regained motor skills to pre-pregnancy level 6 months following delivery. Breast feeding was continued while on ERT. We also report a second patient in whom the diagnosis of Pompe disease was confirmed 5 years after delivery. In review of history the patient was symptomatic since age 15 years. Review of the literature of the published data of pregnancy in Pompe disease with continuation of ERT did not show any adverse outcome for the fetus. We add to the literature, our experience of continuation of ERT throughout the pregnancy and lactation in a patient with Pompe disease. More data needs to be collected to address the understanding of use of ERT during pregnancy and lactation.
Keywords
Lactation; Enzyme replacement therapy; Pregnancy; Pompe disease
Lactation articles; Enzyme replacement therapy articles; Pregnancy articles; Pompe disease articles
Article Details
1. Introduction
Pompe Disease (PD; Glycogen Storage Disease type II,OMIM: 232300) is a rare autosomal recessive lysosomal storage disorder caused by the deficiency of the enzyme acid alpha glucosidase. Deficiency of this enzyme results in accumulation of lysosomal glycogen in skeletal, cardiac and smooth muscles. Phenotypic spectrum of this disease ranges from the classic infantile form presenting with Hypertrophic Cardiomyopathy (HCM) in early infancy leading to death by 1 year of age to Late Onset Pompe Disease (LOPD) manifesting as early as the first year of life to patients presenting as late as the 6th decade of life. LOPD can have presentation as childhood, juvenile or adult onset forms. The distinguishing feature of LOPD is the absence of cardiomyopathy in first year of life. Since the advent of newborn screening (NBS), incidence of PD is reported to be 1 in 16,095 which includes infantile and LOPD [1]. Patients with LOPD present with a slowly progressive limb girdle weakness, respiratory failure and hyperCKemia with little/no cardiac involvement. LOPD primarily affects limb girdle muscles, diaphragm and paraspinal muscles. Respiratory failure is the most common cause of death in patients with LOPD. Diaphragmatic muscle weakness can occur even before obvious involvement of proximal muscle weakness [2]. Weakness of paraspinal muscles is an important clue manifesting with the difficulty in sitting up from the supine position. The other manifestations in LOPD include scoliosis, osteoporosis, sleep apnea, chronic fatigue and intracranial aneurysms [2]. Due to the variability of clinical presentations there should be a high index of suspicion while evaluating patients with proximal muscle weakness for early identification of LOPD. Puri et al. have reported the average time delay from the onset of symptoms to diagnosis to be more than 5 years in a cohort of 20 patients with LOPD from India [2]. Recombinant Human Acid Alpha-Glucosidase (rHGAA) was approved as Enzyme Replacement Therapy (ERT) for all forms of PD by FDA and EMEA in 2006. The beneficial effect of ERT on preventing the deterioration of respiratory function and the mortality rate in treated patients was almost five times lower than that in untreated patients and in LOPD patients and it provides improvement and or stabilization of motor function [3]. Better results are achieved for patients in whom the therapy has been initiated at an early age prior to progressive muscle damage. Whilst, the data on response to therapy in terms of skeletal and respiratory involvement is increasing, there is limited knowledge on pregnancy outcome, management during pregnancy and safety of ERT during pregnancy and lactation. There are case reports of pregnancy outcomes of 13 patients with LOPD for whom rHGAA has been continued throughout pregnancy and in the postpartum period [3-11]. The effect of ERT on uterine smooth muscle in PD is not well understood. Animal studies have shown that acid alpha-glucosidase is not transported across the placenta and therefore probably not harmful to the fetus [3]. ERT with rHGAA for PD has been classified as category C by FDA and as per the label, the use of rHGAA during pregnancy should be weighed against the potential benefits and risks [4]. This first case report from India describes the clinical course of PD in a 21 year old woman, with early clinical presentation from 10 years who had a successful pregnancy outcome under continued ERT throughout her pregnancy and in the postpartum period.
1.1 Patient
The proband from Southern state of Kerala in India is the first in birth order born to non-consanguineous couple. At age 10 years, she noticed progressive difficulty to get up from squatting position and difficulty in climbing stairs. By the age of 13 years, she required support to climb stairs and also had frequent buckling of knees with a tendency to fall. She was evaluated at the age of 16 years and was found to have bilateral calf muscle hypertrophy and a positive Gower’s sign. Her Creatine Kinase (CK) level was 2427 U/L (<180 U/L). With the clinical presentation and elevated CK, a possibility of Juvenile PD was considered. Her cardiac evaluation and echocardiogram was normal; Pulmonary Function Test (PFT) at sitting posture was 56%. Enzyme analysis of acid alpha glucosidase in leucocytes, revealed a very low value 2.3 nmol/hr/mg (56-296 nmol/hr/mg), which was consistent with the clinical diagnosis of PD; molecular analysis identified a compound heterozygous mutation, c.1A>G (p.M1V) in exon 2 (initiator mutation; reported) and c.1792A>T (p.I598F) in exon 13 (missense; novel) in GAA. Her parents were investigated and found to be heterozygous carriers for each of these above mentioned mutation variants; her younger brother did not carry any of these mutations. She was started on ERT with recombinant human alglucosidase alfa (rHGAA, Myozyme©) at 20mg/kg every 2 weeks at the age of 16 years. Access to ERT was provided by Sanofi Genzyme as part of its INCAP (India Charitable Access program). She started showing progressive improvement and reduction in the frequency of falls, she was able to climb stairs with minimal support approximately 10 months after initiation of rHGAA infusions. Forced Vital Capacity (FVC) in sitting posture was 55.5%. She got married at 21 years of age and conceived spontaneously, after completing 5 years of ERT. She was followed up closely under the multidisciplinary team comprising of obstetrician, geneticist, pulmonologist, and cardiologist. She was advised to consume a high protein and low carbohydrate diet during pregnancy and ERT was continued throughout her pregnancy. PFT was monitored in the upright and supine posture, in the first trimester and third trimester, which showed no significant decrement in the FVC and were 52% in first trimester and 49% in the third trimester in both postures. Nevertheless, FVC values in first and third trimester were less than 55% of predicted expected levels for patients with PD.
2D Echocardiography was repeated twice during pregnancy which showed a trivial mitral and tricuspid regurgitation with a left ventricular ejection fraction of 60%. She was noted to develop significant worsening of myopathy during third trimester with difficulty to get up from the chair and needing support to climb stairs but did not have respiratory compromise at any time. Prenatal diagnosis was not offered as her spouse was not carrying a pathogenic variant in the GAA gene. In view of the worsening muscle weakness, she underwent an elective caesarean section at 36 weeks of gestation. Spinal anaesthesia was given with 1.8ml 0.5% heavy bupivacaine and 10µg fentanyl after confirming the level of block upto T6. She delivered a female baby, weighing 2800 gm with an APGAR of 8/9 at 1 minute and 5 minutes respectively. Oxytocin infusion was started following the delivery. Postoperative analgesia was provided with ultrasound-guided bilateral transverse abdominus plane block. The intraoperative and postoperative periods were uneventful. Deep vein thrombosis prophylaxis was given preoperatively and postoperatively. She was continued on ERT and has currently completed 120 infusions. Breastfeeding is being continued and she was advised to defer breast feeds for 24 hours from the start of infusion as the high levels of enzyme activity was found to return to normal levels by 24 hours following infusion (5). Six months after delivery, she has regained her muscle power as in the pre-pregnancy period but has a waddling gait with exaggerated lumbar lordosis and the infant has a normal development.
1.2 Patient
The proband is the second in birth order born to a non-consanguineous couple. She was apparently normal till 15 years of age, when she developed a waddling gait. By 17 years of age, she had progressive difficulty to climb stairs and to get up from the squatting position. However, she did not have any significant dyspnoea. She was married at the age of 20 years and had a spontaneous conception, after 5 months of marriage. During the course of her pregnancy, she was noted to have remarkable proximal muscle weakness with frequent buckling of knees and difficulty to climb stairs during the third trimester. She delivered a female baby via caesarean under regional anaesthesia due to transverse lie of the fetus. Following delivery, her proximal muscle weakness worsened with increased frequency of falls with significant difficulty to get up from the floor. She also noticed difficulty to lift heavy objects from the floor, raise upper limb above the level of head and difficulty to get up from supine position. At the age of 25 years, she was evaluated and her CK levels were 1479 U/L (<180 U/L), aspartate Aminotransferase (AST) 142 IU/L (5-40 IU/L) and alanine transaminase (ALT) 176 IU/L (7-56 IU/L). Muscle biopsy showed features of a vacuolar myopathy suggesting the possibility of glycogen storage disease. Enzyme analysis of acid alpha glucosidase from leucocytes revealed levels 2.3 nmol/hr/mg (56-296 nmol/hr/mg) confirming the diagnosis of PD and was referred to the genetic department. Genetic analysis identified a reported compound heterozygous missense mutations, c.1942G>A(p.Gly648Ser) in exon 14 and c.2173C>T(p.Arg725Trp) in exon 15 in GAA. Echocardiography evaluation showed left atrial dilatation due to stiff left ventricle and left ventricular ejection fraction was 60%. Electrocardiogram showed ectopic atrial rhythm. ERT with rHGAA (Myozyme©) was initiated at 26 years at a dose of 20mg/kg as intravenous infusion fortnightly. After 20 infusions, she did not have any falls and could get up from the chair with less effort, and after 80 infusions, she could climb stairs without support. PFT was sequentially monitored, both in the upright and supine posture, which revealed a stable pattern in the FVC over the span of 5 years after initiation of ERT (Figure 1).Currently, she has completed 146 infusions over the past 6.5 years. Presently, she can do all household chores comfortably, although she has mild waddling gait and exaggerated lumbar lordosis.
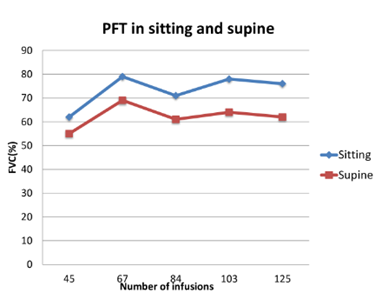
Figure 1: Pulmonary Function Test (PFT) in patient 2 over a 5 year period showing a steady pattern of Forced Vital Capacity (FVC) in sitting and supine position.
2. Discussion
Pregnancy, in general, is physically demanding and even more so for women with LOPD and considered as high risk as pregnancy may worsen symptoms, or may initiate symptoms of PD thereby leading to confirmation of the diagnosis. In addition to the stress associated with physiological changes of pregnancy, patients with PD have additional burden due to myopathy and diaphragmatic muscle involvement leading to further compromise with advancement of gestation. Complication rates in pregnancy or child birth are not reported to be increased in patients with PD except for a small increased rate of still births and anaesthetic complications [6]. Karabul et al reported the obstetric outcome of 66 patients with PD. Majority of women became pregnant prior to the confirmation of diagnosis as PD and among the cohort who were already diagnosed with PD, there was no higher risk of developing complications either during pregnancy or delivery [7]. Women who are already diagnosed with PD have experienced worsening of muscle weakness and respiratory symptoms during pregnancy and improvement in the postpartum period [6,7]. There is a considerable risk of not recovering to the pre pregnancy motor and respiratory level after delivery [8]. Previous studies have shown that most of the women with PD had confirmation of diagnosis after the completion of all pregnancies [7]. There are 13 patients with LOPD in whom the prospective data are available regarding their pregnancy outcome while on ERT (Table 1). Previous experiences also have not shown any adverse events in patients with LOPD who have continued ERT throughout their pregnancy [3-14]. Our patient is the first patient with LOPD reported from India who had a successful pregnancy outcome along with continuation of ERT throughout her pregnancy and in the postpartum period. She had presented with proximal muscle weakness by 10 years of age. Like other LOPD patients reported in the Indian cohort by Puri et al, she too did not have the common leaky splice site LOPD variant, c. −32 −13T > G (IVS1) mutation. She was a compound heterozygote and one of her mutations was an initiator mutation which is a common mutation seen among patients with infantile PD and her second one was a novel missense mutation. She experienced worsening of muscle weakness in the third trimester while on ERT. Her pulmonary status remained stable. She regained her pre pregnancy muscle power six months following delivery and could carry out the household chores that she has been carrying out before becoming pregnant. The course of our patient is similar to what has been reported in the literature. They had worsening of motor and respiratory function especially in the third trimester but had regained their muscle strength within a year after delivery [3, 8, 9]. This could be due to the additional stress due to weight gain during the pregnancy (Patient 1 had gained 11.5kg) and metabolic and hormonal changes in the context of an underlying myopathy. Interruption of ERT during pregnancy has been associated with deterioration of motor and respiratory symptoms in previously stable patients [8]. The reasons reported in the literature for discontinuation of ERT during first trimester of pregnancy were 1.High dose of GAA (20mg/kg every 2 weeks) which is recommended for PD in comparison with Gaucher and Fabry disease (20 fold higher), 2.concerns about the potential harmful effects of GAA or its components to the developing fetus, 3.potential drug related hypersensitivity reactions [4, 8].
Table 1: Pregnancy outcomes in LOPD patients following continuation of ERT during pregnancy.
According to the current literature from sporadic case reports, continuation of ERT during pregnancy seems to be safe and also important to prevent deterioration of clinical status of the mother with no apparent adverse effects to the developing fetus. On the contrary cessation of ERT in early pregnancy have resulted in deterioration of muscle power and respiratory function and increased risk of allergic reactions on recommencing ERT [4]. Back pain has been reported as the most common Pompe related symptom during pregnancy and 84% of the subjects had back pain as the predominant symptom among a cohort of 25 pregnant women with PD [6]. During pregnancy the use of high protein diet helps to overcome the muscle weakness [10]. Pregnant women with LOPD are also advised to consume folic acid and vitamin D and to avoid vitamin A supplements and to limit intake of oily fish [4]. In women with neuromuscular disorders, a FVC < 50% predicted (< 1L) at baseline should be monitored very closely during pregnancy and delivery in view of associated maternal hypoxia [7]. PFT should be done in each trimester and arterial blood gas sampling and respiratory sleep study should be offered to those patients showing a decreasing trend of FVC. Maternal hypoxia resulting in oxygen saturation less than 85% leads to live birth rates as low as 12% and this risk factor should be avoided [6,11]. PFT usually show a restrictive pattern in PD. Diaphragmatic weakness is evaluated by comparing the FVC in upright and supine postures. Diaphragmatic weakness is suggested if there is a >10% decrease of FVC in the supine compared to upright position and a >30% decrease indicates severe weakness [2]. Patient 1 had a low FVC during first and second trimester during upright and supine posture but there was no significant fall in FVC in supine posture. As she is currently asymptomatic and does not have exertional dyspnoea or early morning headache, she has not been initiated on nocturnal mechanical ventilatory support. She underwent PFT evaluation eight months after delivery, and the upright and supine values are 52% and 48% respectively which are remaining stable as in the pre pregnancy period. Patient 2 had shown a stable pattern of FVC over the past 5 years while on ERT. Longitudinal FVC data from LOPD patients from Pompe registry had shown that stability of FVC for 5 years is due to the effect of long-term ERT [12].
Anaesthetic team should be involved along with the multidisciplinary team consisting of obstetrician, geneticist, pulmonologist and cardiologist from early gestation. Caesarean section is indicated only for obstetric reasons or medical reasons which are related to PD [4]. Spinal anaesthesia is the technique of choice as it circumvents the respiratory and airway complications. Other anaesthetic choices include epidural or combined spinal-epidural anaesthesia. Spinal or epidural anaesthesia might be extremely difficult or impossible in a patient with severe scoliosis. Should general anaesthesia be needed, the anaesthetist should be aware of the possible need for prolonged postoperative mechanical ventilation [10]. As they are prone for aspiration, the airway needs to be protected with a cuffed endotracheal tube. Depolarising muscle relaxant, suxamethonium chloride should be avoided as it can cause severe hyperkalemia [10]. A reduced dose of non-depolarizing neuromuscular blockers is sufficient in these patients. Careful or restricted use of opioids is recommended to avoid postoperative respiratory complications. Patient 1 and 2 underwent caesarean section under spinal anaesthesia without facing any adverse effects. There is only one previous report of a patient with PD who had continued breast feeding while on ERT [5]. Patient 1 exclusively breast fed her baby and was advised to stop feed for 24 hours from the start of each infusion. de Vries et al have performed pharmacokinetic studies on breast milk following ERT to estimate the amount of infused rHGAA secreted through breast milk. Activity levels in breast milk had peaked at 2.5 hours after the end of infusion and this was 2 hours later than the plasma peak. Enzyme activity in breast milk was back to pre-infusion level by 24 hours after the start of the infusion [5]. This observation is parallel to secretion of Imiglucerase levels in breast milk in women with Gaucher disease while on ERT. More prospective studies are needed to prove the safety of ERT throughout pregnancy for maintaining the motor and respiratory function and for continued use in the postpartum period so that breast feeding can be recommended safely.
Conclusion
Pregnancy in a woman with PD should be considered as potentially high-risk, and requires special attention and monitoring. According to the current literature and our own experience, continuing ERT during pregnancy in patients with PD might be a beneficial option to maintain muscular and pulmonary functions with no apparent adverse effects to the developing fetus particularly in patients requiring assisted ventilation. Even though there is scarce data regarding the safety of continuation of ERT during lactation, the available literature have not shown any adverse events in infants with continuation of breast feeds.
Acknowledgements
We express our gratitude to Sanofi Genzyme for supporting patients with Pompe disease with enzyme replacement therapy under the INCAP (India charitable access programme)
Funding
None
Conflict of Interests
None declared
Ethics Approval
Ethical approval for this study was granted by the Ethics (Medical Research) Committee Office, Amrita Institute of Medical Sciences & Research Centre, Cochin, Kerala, India
Patient Consent
Not required
References
- Ficicioglu C, Ahrens-Nicklas RC, Barch J, et al. Newborn Screening for Pompe Disease: Pennsylvania Experience. Int J Neonatal Screen 6 (2020): 89.
- Puri RD, Setia NNV, Jagadeesh S, et al. Late onset Pompe Disease in India - Beyond the Caucasian phenotype. Neuromuscul Disord 3 (2021): 431-441.
- Holbeck-Brendel M, Poulsen BK. Treatment with enzyme replacement therapy during pregnancy in a patient with Pompe disease. Neuromuscul Disord 27 (2017): 956-958.
- Rohman PJ, Scott E, Richfield L, et al. Pregnancy and associated events in women receiving enzyme replacement therapy for late-onset glycogen storage disease type II (Pompe disease). J Obstet Gynaecol Res 42 (2016): 1263-1271.
- de Vries JM, Brugma JD, Ozkan L, et al. First experience with enzyme replacement therapy during pregnancy and lactation in Pompe disease. Mol Genet Metab 104 (2011): 552-555.
- Goker-Alpan O, Kasturi VG, Sohi MK, et al. Pregnancy Outcomes in Late Onset Pompe Disease. Life (Basel) 10 (2020): 194.
- Karabul N, Berndt J, Kornblum C, et al. Pregnancy and delivery in women with Pompe disease. Mol Genet Metab 112 (2014): 148-153.
- Santos MO, Evangelista T, Conceicao I. Enzyme replacement therapy with alglucosidase alfa in a late-onset Pompe disease patient during pregnancy. Neuromuscul Disord 28 (2018): 965-968.
- Zagnoli F, Leblanc A, Blanchard C. Pregnancy during enzyme replacement therapy for late-onset acid maltase deficiency. Neuromuscul Disord 23 (2013): 180-181.
- Cilliers HJ, Yeo ST, Salmon NP. Anaesthetic management of an obstetric patient with Pompe disease. Int J Obstet Anesth 17 (2008): 170-173.
- Perniconi B, Vauthier-Brouzes D, Morélot-Panzini C, et al. Multidisciplinary care allowing uneventful vaginal delivery in a woman with Pompe disease. Neuromuscul Disord 26 (2016): 610-613.
- Stockton DW, Kishnani P, van der Ploeg A, et al. Respiratory function during enzyme replacement therapy in late-onset Pompe disease: longitudinal course, prognostic factors, and the impact of time from diagnosis to treatment start. J Neurol 267 (2020): 3038-3053.
- Klos J, Kwasniak-Butowska M, Slawek J. Alglucosidase alfa therapy for Pompe disease in pregnancy - Case report. J Neurol Sci 375 (2017): 167-169.
- Van HoutteJ, De Bleecker JL. Two successfully completed pregnancies in adult onset Pompe disease, under continued treatment with alglucosidase alfa. Acta Neurol Bel 119 (2019): 147-149.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
