Recurrent Squamous Cell Carcinoma Arising from Ovarian Mature Cystic Teratoma with Cardiac Metastases: A Case Report
Article Information
Lixia Cai1, Ling Wang1, Xiangyu Chen1*, Zhelan Zheng2
1Department of Ultrasound, Zhuji People's Hospital of Zhejiang Province, Zhuji Affiliated Hospital of Shaoxing University, China
2Department of Ultrasound, The First Affiliated Hospital of Zhejiang University, China
*Corresponding Author: Xiangyu Chen, Department of Ultrasound, Zhuji People's Hospital of Zhejiang Province, Zhuji Affiliated Hospital of Shaoxing University, China
Received: 07 March 2022; Accepted: 22 March 2022; Published: 31 March 2022
Citation: Lixia Cai, Ling Wang, Xiangyu Chen, Zhelan Zheng. Recurrent Squamous Cell Carcinoma Arising from Ovarian Mature Cystic Teratoma with Cardiac Metastases: A Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 234-242.
View / Download Pdf Share at FacebookAbstract
Introduction: Mature Cystic Teratoma (MCT) is a common ovarian germ cell tumor, the secondary development of malignancy in MCT is rare. Squamous-Cell Carcinoma (SCC) accounts for 80% of secondary malignant transformations of MCT (SCC-MCT). SCC-MCT with cardiac metastases is extremely rare.
Patient concerns: we report a 35-year-old Chinese woman who chose to undergo fertility sparing surgery due to her second childbirth needs. 2 years later, SCC-MCT recurred with cardiac metastases.
Diagnoses: Postoperative pathology confirmed that the cardiac tumor was poorly differentiated squamous cell carcinoma derived from the ovary.
Interventions: Patient underwent chemotherapy after cardiac tumor resection.
Outcomes: Patient requested to be discharged after chemotherapy, no follow-up information available.
Lessons: Due to the difficulty of preoperative diagnosis and poor prognosis, it is necessary to be cautious when choosing fertility-sparing surgery for SCC-MCT.
Keywords
Cystic Teratoma; Chemotherapy; Fertility; Squamous-cell; Uterus
Cystic Teratoma articles; Chemotherapy articles; Fertility articles; Squamous-cell articles; Uterus articles
Article Details
Abbreviations:
IVC: Inferior vena cava; M: Mass; MCT: Mature cystic teratoma; RA: Right atrium; RV: Right ventricle; SCC: Squamous-cell carcinoma; TV: Tricuspid valve; UT: Uterus
1. Introduction
Mature Cystic Teratoma (MCT) is a common ovarian germ cell tumor, accounting for about 10-20% of ovarian tumors [1]. Malignant Transformation (MT) occurs in about 0.17-2% of MCT [2]. Squamous Cell Carcinoma (SCC) arising from MCT (SCC-MCT) is the most common malignant transformation (about 80%) [3], mostly in postmenopausal women, rare in women of childbearing age [4]. Due to the lack of specific clinical symptoms, it is usually discovered accidentally during gynecologic investigations for other conditions [5]. Imaging modalities and laboratory tests are not specific, either [6]. Therefore, preoperative diagnosis is very difficult. Meanwhile, SCC-MCT with cardiac metastases is even rarer. Here we report a case of SCC-MCT (International Federation of Gynecology and Obstetrics stage FIGO Ia) in a woman of childbearing age who recurred with cardiac metastases two years after undergoing fertility-sparing surgery. Through the literature review, we focus on clinicopathological characteristics, methods of early prediction for the disease, and fertility- sparing treatments.
2. Case Presentation
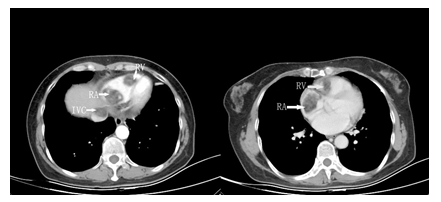
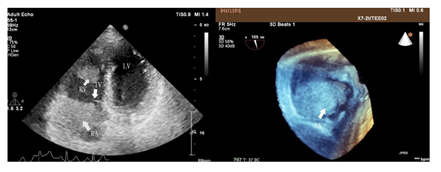
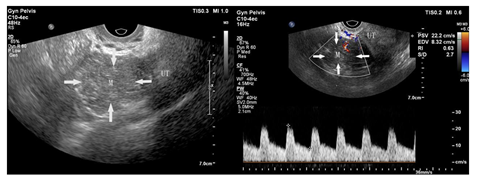
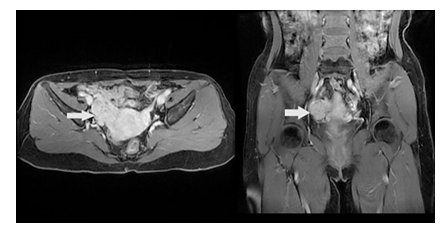
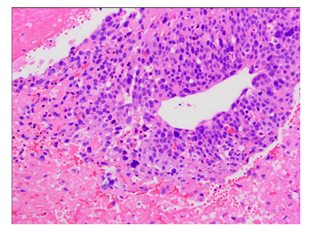
A 35-year-old Chinese woman-gravida 1, para 1presented with a 2weeks history of palpitations, worsening cough and dyspnea on exertion for 1week. 2 years prior to this visit, she underwent surgical treatment for SCC-MCT (FIGO Ia). In consideration of patient's desirement for second child birth, only the right salpingo-oophorectomy was performed without chemotherapy. Upon examination, her blood pressure was 106 / 80mmHg and body temperature was 37.0°C, The respiratory rate was 29 times/ minute. Her oxygen saturation was 95%. Heart auscultation revealed a 3/6 grade systolic murmur in the tricuspid area. The Physical examination revealed slight edema in bilateral lower extremity. The white blood cell count was 6.2 × 109 /L, neutrophils (%) (68.5%), the hemoglobin was 124 g/L. Her creatinine and transaminase were normal. The tumor marker levels were as follows: CA125: 93 U/mL (<35 U/mL), CA19–9: 63 U/ml (<27 U/mL), and carcinoembryonic antigen (CEA): 14 ng/mL (<2.5 ng/mL), squamous cell carcinoma antigen (SCCA): 7.2 mg/L (<1.5mg/L), AFP: 3.3ng/ml (<15ng/ml). Her HIV test was negative, as was hepatitis B and C. The electrocardiogram showed sinus rhythm with a heart rate of 86 beats per minute. Chest CT detected low-attenuating lesions in the right atrium, right ventricle and inferior vena cava, no metastasis was found in the lungs. Whether during the arterial phase or the delayed phase, the masses demonstrated heterogeneous mild enhancement (Figure1). Transthoracic echocardiography found that the right atrium tumor was adjacent to right ventricular inflow tract, resulting in obstruction of the tricuspid valve (Figure2). Left ventricular ejection fraction is in the normal range. Transvaginal ultrasound revealed hypoechoic echo mass in right pelvic cavity with arterial blood flow signal (Figure3). Contrast-Enhanced Magnetic Resonance Imaging (CE-MRI) showed moderate enhancement of the right pelvic mass (Figure4). Thrombosis was not seen in lower extremity vascular ultrasonography. No obvious metastasis was found in other organs by PET- CT. Based on the results of all examinations and tumor history, the patient was diagnosed with recurrent SCC-MCT with cardiac metastases. In order to alleviate the hemodynamic impairment and provide an opportunity for chemotherapy, we performed emergency surgery to remove the masses in the heart and inferior vena cava. Filter implanted in the inferior vena cava to prevent cancerous emboli from forming again. Pathological examination showed squamous cell carcinoma with massive necrosis (Figure5). Subsequently, the patient underwent ultrasound-guided pelvic mass biopsy. On microscopic examination, the lesions were identified as poorly-differentiated squamous cell Carcinoma. To further inhibit the rapid growth of tumors, combination of paclitaxel and carboplatin was adopted. After completion of chemotherapy, patient was discharged home.
3. Discussion
Mature Cystic Teratomas (MCTs) are very common in women of childbearing age and occur bilaterally in 10-17% of patients [7]. MCTs with malignant transformation are very rare, accounting for only 2% to 4% of all cases, and are usually observed in postmenopausal women, rare in women of childbearing age [8]. According to the most recent Surveillance, Epidemiology and End Results (SEER) cancer statistics report based on the data from 2012 to 2016, the percentages for newly diagnosed, ovarian cancer under the age of 45 are 12% [9]. Intertwined with the increasing sociodemographic transition toward women having first childbirth beyond age 35, reproductive aging, and gonadotoxic treatments, fertility issues have become more prevalent and complicated in women with ovarian cancer [10]. In most patients, SCC-MCTs are asymptomatic and few patients have more than one symptom related to the tumor. Tumor of early stage is often detected accidentally during physical examination or postoperative pathological examination, while palpable abdominal or pelvic mass, abdominal pain and distention are often present in advanced stage [11]. Acute abdomen may occur due to tumor torsion or rupture [12]. Recurrence after fertility-sparing surgery with cardiac metastasis has not been reported. MCT can be easily diagnosed by imaging investigation such as gynecological sonography, CT and MRI, however, SCC-MCT is difficult to predict or diagnose from imageological results [13]. Moreover, tumor mark tests are not specific, either. Squamous-Cell-Carcinoma Antigen (SCCA), CA125, CA19-9 and CEA are raised in many patients with SCC-MCT. However, high concentrations of tumor markers have been reported in patients with benign tumors [14, 15]. CA19-9 and CA125 measurement did not allow distinction between malignant and non-malignant MCTs [16]. Serum concentrations of SCCA was not related to the stage of disease but rather with adverse prognosis [2]. Intraoperative diagnosis of squamous-cell carcinoma arising in a mature cystic teratoma might be difficult. In one study, malignant disease was detected in frozen tissue sections in only 50% of cases [17], therefore, MCT in women older than 30 years that show unusual adherence and suspect solid areas or firm, friable, myxomatous or variegated areas should arouse suspicion [18]. The current clinical studies were retrospective due to the low incidence of SCC-MCT and the published cases are scattered, and the optimal treatment for SCC transformation in MCT remains unclear [19].
The standard primary surgery should consist of bilateral salpingo-oophorectomy, total hysterectomy and comprehensive surgical staging (peritoneal washing, omentectomy, appendectomy, peritoneal biopsies, and pelvic plus paraaortic lymphadenectomy) in early disease and optimal cytoreductive surgery in advanced disease [20]. Hysterectomy and bilateral salpingo- oophorectomy appear to improve survival of SCC-MCT. Fertility-sparing surgery usually is safe and feasible in patients with fertility needs under the age of 45 in the IA / IC stage [21]. In Yoshikawa's study, 5-year overall survival of patients undergoing fertility-preservation surgery were likely to be equivalent with those of patients who underwent radical surgery [22]. However, Hyun Nam et al. reported that young women with early-stage ovarian cancer undergo fertility sparing surgery with 5.2% mortality and 11% recurrence [23]. Because of poor prognosis, the risk of recurrence caused by the choice of conservative surgery should be fully informed to patients. Compared with stage Ia patients who only need follow-up observation after surgery, chemotherapy in advanced patients is necessary. There is no recognized first-line adjuvant therapy for SCC-MCT yet. Currently the combination of paclitaxel and carboplatin is the most commonly adopted regimen [1]. Postoperative radiotherapy did not improve patient’s survival [6]. Nowadays, increasing number of women are delaying their childbearing age to over 35. Preservation of reproductive function is strongly desired by patients with ovarian cancer at childbearing age [24]. Following comprehensive surgery and adjuvant chemotherapy, women are at increased risk of experiencing ovarian insufficiency and early menopause, as well as fibrosis, atrophy, and vascular injury in reproductive organs due to surgical injury and drug toxicity. Prior to comprehensive surgery and adjuvant chemotherapy, it is feasible and necessary to use assisted reproduction technology to preserve the fertility of patients. Controlled Ovarian Hyperstimulation (COH) cycle followed by oocyte and embryo cryopreservation can be successfully performed to preserve fertility. A major concern regarding the conventional COH protocols is the elevated circulating estradiol levels due to the development of multiple large follicles at once that can worsen oncological outcome in estrogen-sensitive cancers. Hence, Oktay et al. have developed safer ovarian stimulation protocols that provide high oocyte and embryo yields using anti-estrogenic drugs such as Tamoxifen and Letrozole, which can be used alone or in combination with lower doses of gonadotropins [25].
However, when there is no sufficient time for a COH cycle, patients can be offered ovarian tissue cryopreservation (OTC) and subsequent autotransplantation (OvTx) procedure [26]. There are some promising advances that can be clinically applied to prevent ovarian damage caused by chemotherapy in the future, such as antiapoptotic/cell preserving agents [27], stem cell technologies [28], in vitro oogenesis using induced pluripotent stem cells (iPSCs) [29], in vitro primordial follicle growth [29] and 3-D printed ovarian matrices to be populated by human primordial follicles (“artificial ovary”) [30].
It is extremely important to promptly referred to a reproductive specialist on which assisted reproductive technology is adopted to preserve fertility. However, Selter et al. recently reported that in 2016 only 5.5% of reproductive age women with lung, breast, colorectal or cervical cancer was evaluated for fertility preservation in the United States, and only 4.6% of those patients underwent a fertility preservation procedure [31]. An individualized multidisciplinary approach and timely referral to a reproductive specialist is crucial for achieving best results for SCC-MCT patients desiring to preserve their fertility. SCC-MCT is a rare malignancy. Because of the difficulty of preoperative diagnosis and poor prognosis, it is necessary to be cautious when choosing fertility-sparing surgery. Patients facing the potential infertility due to oncological treatments should be promptly referred to a reproductive specialist. More clinical trials are in need to elucidate the best treatment options for SCC-MCT.
Conflict of Interest
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Ethical Statements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from the patient for publication of this case report.
References
- Gadducci A, Guerrieri ME, Cosio S. Squamous cell carcinoma arising from mature cystic teratoma of the ovary: A challenging question for gynecologic oncologists. Crit Rev Oncol Hematol 133 (2019): 92-98.
- Hackethal A, Brueggmann D, Bohlmann MK, et al. Squamous- cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. The Lancet Oncology 9 (2008): 1173-1180.
- Chiang AJ, Chen MY, Weng CS, et al. Malignant transformation of ovarian mature cystic teratoma into squamous cell carcinoma: a Taiwanese Gynecologic Oncology Group (TGOG) study. J Gynecol Oncol 28 (2017): 69.
- Kim MJ, Kim NY, Lee DY, et al. Clinical characteristics of ovarian teratoma: age- focused retrospective analysis of 580 cases. American journal of obstetrics and gynecology 205 (2011): 32.
- Choi EJ, Koo YJ, Jeon JH, et al. Clinical experience in ovarian squamous cell carcinoma arising from mature cystic teratoma: A rare entity. Obstetrics & gynecology science 57 (2014): 274-280.
- Li C, Zhang Q, Zhang S, et al. Squamous cell carcinoma transformation in mature cystic teratoma of the ovary: a systematic review. BMC Cancer 19 (2019): 217.
- Ulbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Modern pathology: an official journal of the United States and Canadian Academy of Pathology Inc 18 (2005): 61-79.
- Oranratanaphan S, Khemapech N. Characteristics and treatment outcomes of patients with malignant transformation arising from mature cystic teratoma of the ovary: experience at a single institution. Asian Pacific journal of cancer prevention: APJCP 14 (2013): 4693-4697.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016. National Cancer Institute (2018).
- Bhasin S, Kerr C, Oktay K, et al. The Implications of Reproductive Aging for the Health, Vitality and Economic Welfare of Human Societies. The Journal of clinical endocrinology and metabolism 16 (2019): 00315.
- Gooneratne AT, James AO, Gupta J, et al. Squamous cell carcinoma arising in a mature cystic teratoma invading the sigmoid colon: a rare presentation. BMJ case reports 2015 (2015).
- Park JY, Kim DY, Kim JH, et al. Malignant transformation of mature cystic teratoma of the ovary: experience at a single institution. European journal of obstetrics, gynecology, and reproductive biology 141 (2008): 173-178.
- Feng X, Xu L. Rare case of squamous cell carcinoma arising in a recurrent ovarian mature cystic teratoma of a young woman: A case report and review of the literature. Medicine (Baltimore) 97 (2018): 10802.
- Nagata H, Takahashi K, Yamane Y, et al. Abnormally high values of CA 125 and CA 19-9 in women with benign tumors. Gynecologic and obstetric investigation 28 (1989): 165-168.
- Dede M, Gungor S, Yenen MC, et al. CA19-9 may have clinical significance in mature cystic teratomas of the ovary. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society 16 (2006): 189-193.
- Kikkawa F, Ishikawa H, Tamakoshi K, et al. Squamous cell carcinoma arising from mature cystic teratoma of the ovary: a clinicopathologic analysis. Obstetrics and gynecology 89 (1997): 1017-1022.
- As AK, Webb JB, Wijesinghev D. Advanced squamous cell carcinoma developing in a mature cystic teratoma (dermoid) of the ovary in a young woman: a diagnostic and therapeutic challenge. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 17 (1997): 598-599.
- Stirling D, Evans DG, Pichert G, et al. Screening for familial ovarian cancer: failure of current protocols to detect ovarian cancer at an early stage according to the international Federation of gynecology and obstetrics system. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23 (2005): 5588-5596.
- Glasspool RM, Gonzalez Martin A, Millan D, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for squamous cell carcinoma of the ovary. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 24 (2014): 26-29.
- Balik G, Ustuner I, Bedir R, et al. Appendix and uterus metastasis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Case reports in obstetrics and gynecology 2013 (2013): 474891.
- Budiman HD, Burges A, Ruhl IM, et al. Squamous cell carcinoma arising in a dermoid cyst of the ovary in pregnancy. Archives of gynecology and obstetrics 281 (2010): 535-537.
- Yoshikawa N, Teshigawara T, Ikeda Y, et al. Fertility-sparing surgery of malignant transformation arising from mature cystic teratoma of the ovary. Oncotarget 9 (2018): 27564-27573.
- Nam JH, Park JY. Fertility-sparing surgery for young women with early-stage epithelial ovarian cancer. Gynecologic and obstetric investigation 76 (2013): 14-24.
- Taylan E, Oktay K. Fertility preservation in gynecologic cancers. Gynecol Oncol 155 (2019): 522-529.
- Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23 (2005): 4347-4353.
- Taylan E, Oktay K. Chapter 30-Autologous Transplantation of Human Ovarian Tissue. In: Leung PCK, Adashi EY, eds. The Ovary (Third Edition). Academic Press (2019):493-500.
- Li F, Turan V, Lierman S, et al. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Human reproduction (Oxford, England) 29 (2014): 107-113.
- White YA, Woods DC, Takai Y, et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nature medicine 18 (2012): 413-421.
- Yamashiro C, Sasaki K, Yabuta Y, et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science (New York, NY) 362 (2018): 356-360.
- Marie-Madeleine D, Christiani AA. FERTILITY PRESERVATION: Construction and use of artificial ovaries. Reproduction 158 (2019): 15-25.
- Selter J, Huang Y, Grossman Becht LC, et al. Use of fertility preservation services in female reproductive-aged cancer patients. American journal of obstetrics and gynecology 221 (2019): 328.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
