Double Coronary Aneurisms in Kawasaki Disease Successfully Treated with Anakinra: A Case Report
Article Information
Bouayed K1*, Lotfi S1, Sakhi A1, Driguil A2
1Department of Pediatric Rheumatology and Internal Medicine, A. Harouchi Mother and Child Hospital CHU Ibn Rochd, Casablanca, Morocco
2Department of Cardiology, CHU Ibn Rochd, Casablanca, Morocco
*Corresponding Author: Bouayed K, Department of Pediatric Rheumatology and Internal Medicine, A. Harouchi Mother and Child Hospital CHU Ibn Rochd, Casablanca, Morocco.
Received: 16 November 2022; Accepted: 29 November 2022; Published: 30 December 2022
Citation: Bouayed K, Lotfi S, Sakhi A, Double Coronary Aneurisms in Kawasaki Disease Successfully Treated with Anakinra: A Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 780-783.
View / Download Pdf Share at FacebookAbstract
Background: Kawasaki disease is an acute inflammatory vasculitis of the medium and small-caliber arteries, usually occurring in children under 5 years of age. It is known to cause coronary aneurysms, which are more frequent in refractory forms. While the treatment of classical Kawasaki disease is well codified, the treatment of refractory forms remains nonstandardized. Anakinra, an interleukin-1 receptor antagonist, appears to be a promising therapeutic alternative.
Case Presentation: We report a case of 9 months old girl, diagnosed with Kawasaki disease, non-responsive to two doses of intravenous immunoglobulins and acetyl salicylic acid, who developed aneurysms, successfully treated with Anakinra 3 months with 5 years of follow-up.
Conclusions: Our experience highlights Interleukin-1 blockade effectiveness in reducing Kawasaki disease systemic inflammation. We believe that our case adds more evidences to the potential role of interleukin-1 receptor antagonist as second or third-line therapy in some cases of refractory Kawasaki disease, particularly with severe involvement of coronary arteries.
Keywords
Anakinra; Coronary Arteries; Kawasaki Disease
Article Details
Abbreviations:
CAA- Coronary Artery Aneurysms; IL- Interleukin; IVIG- Intravenous Immunoglobulin; KD- Kawasaki Disease; LCA- Left Coronary Artery; LDCA- Left Anterior Descending Coronary Artery
1. Introduction
Kawasaki disease (KD) is an acute inflammatory vasculitis of the medium and small-caliber arteries, typically involving coronary arteries, usually occurring in children under 5 years, and represents the leading cause of acquired heart disease in developed countries and the second cause after rheumatic fever in some developing countries. The diagnosis of KD is based on the presence of high fever lasting at least 5 days associated with at least four of the five main features: peripheral extremity involvement, cervical adenopathy, bilateral non-exsudative conjunctivitis, cheilitis, and polymorphic skin rash [1]. The first-line treatment, a single infusion of 2 g/kg intravenous immunoglobulin (IVIG) along with aspirin, reduces coronary artery aneurysms (CAA) frequency from 25–30% to about 3-5% [1,2]. However, 10-20% of patients with KD are resistant to conventional therapy, putting them at significant risk for cardiac complications [3]. The role of interleukin (IL)-1 in the pathophysiology of KD and in the development of CAA is recognized although not well understood. This leads to the potential use of IL-1 blockade as an alternative therapy for refractory KD [4,5]. In this article, we report the case of a 9-month-old girl diagnosed with KD, refractory to two doses of IVIG, who developed aneurysms, successfully treated with Anakinra.
2. Case Report
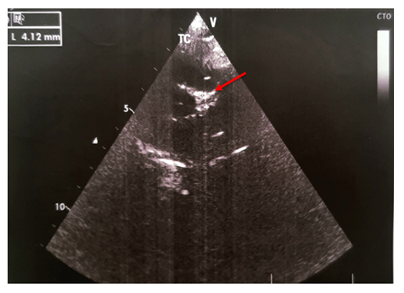
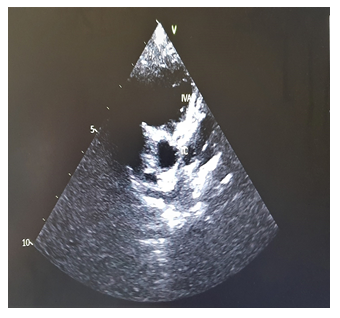
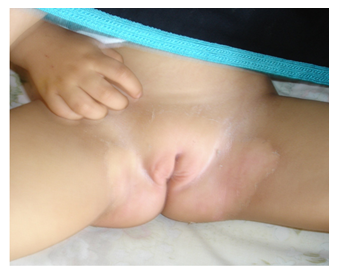
We report the case of a previously healthy 9-month-old girl admitted for 8 days persistent fever at 39.7° on admission. She had asthenia, bilateral non-purulent conjunctivitis, cheilitis , right cervical lymphadenopathy measuring 2 cm and erythematous scaling perineal rash (Photo 1). The blood count cells showed normochromic microcytic anaemia (Hb:9.9 g/dL, MCV: 69 fl, MCHC: 34 g/dL), hyperleukocytosis at 17.3 G/L, hyperneutrophilia at 11.64 G/L, normal platelets at 289 G/L, elevated ESR at 122 mm/1rst hour, increased C-reactive protein at 260 mg/L and subnormal transaminases (AST at 69 U/L, ALT at 33 U/L). The patient fulfilled the criteria of Kawasaki disease. She was treated with intra-venous polyvalent immunoglobulin "IVIG" at a dose of 2 g/kg/perfusion combined with aspirin at 50 mg/kg/d on the 9th day of fever. An echocardiogram performed on the 11th day of fever was normal. The persistence of fever lead to a second echocardiogram on day 16 which revealed double aneurysmal dilatation of the left coronary artery (LCA) to 4.1 mm (Figure 1) and the left anterior descending coronary artery (LDCA) to 3.4 mm justifying a second IVIG infusion on the same day allowing apyrexia. A follow-up echocardiogram was performed on day 24 and showed a worsening of the aneurysm in the LCA to 6 mm and in the LDCA to 4 mm. The inflammatory screening showed a significant increase in platelets to 758 G/L, a persistant inflammatory syndrome with ESR of 40 mm/1st hour and a CRP of 74 g/dL. Due to this extensive coronary lesions and the persistent inflammatory syndrome, third-line treatment with Anakinra 5 mg/kg/day was started for a period of 3 months, combined with aspirin at 5 mg/kg/day leading to regression of the inflammatory syndrome. Subsequent echocardiograms at months 2 and 3 showed decreasing dilatation of the aneurysms with complete normalization of cardio-vascular findings at month 8 allowing discontinuation of aspirin. No changes on coronary arteries size have been recorded over time with a current follow-up of 5 years (Figure 2).
3. Discussion
KD is a systemic vasculitis characterized by increased inflammatory cytokines, such as tumor necrosis factor α, Interleukin (IL) IL-6, and IL-1 [1]. IVIG represents the gold standard treatment and have significantly decreased the incidence of coronary involvement. In contrast, children that do not respond to initial IVIG have a higher risk of developing CAAs [6]. The pathogenesis of IVIG resistance is not well elucidated as the mechanism of action of IVIG is unclear. It appears that host genetic factors, such as polymorphisms in IG-binding gamma Fc receptors or copy number variation of FCGR2C, FCGR3A, and FCGR3B, may be involved in the response and resistance to IVIG [7,8]. Furthermore, an abundance of IL-1α and β-related transcripts has been described in KD blood samples compared to pediatric subjects with different acute infectious diseases or healthy controls [9]. In addition, decreased IL-1 receptor antagonist expression has been reported in IVIG-resistant KD patients [10]. All this suggests that IL-1 excess seems to play a role in the occurrence of KD and in IVIG resistance. Thus, IL-1 blockade represents an attractive target because of its strong role in the pathogenesis of KD and CAAS [5]. Few previous studies reported the use of Anakinra in refractory KD cases; in most patients, it has been used as rescue therapy subsequently to the failure of multiple therapeutic strategies [11]. Anakinra administration was preceded or associated to further IVIG doses [12], Methylprednisolone pulses [11-13] Infliximab [12,14] and Cyclophosphamide [13]. Anakinra appeared to be effective in achieving prompt defervescence and significant reduction of inflammatory markers [11,15,16]. Anakinra treatment showed a complete or partial improvement in most patients with KD who developed coronary complications [12-17]. The first report of anti-IL-1 use in KD dates back to 2012 and described a 2-year-old boy with classic KD who developed myocarditis, coronary artery inflammation and dilatation of the left descending coronary and right coronary arteries, despite 2 doses of IVIG and three boluses of methylprednisolone, who dramatically improved with Anakinra and normalized his cardiac ultrasonography after 6 months [11]. Several other cases have been published reporting the efficacy of Anakinra in refractory KD complicated by severe coronary aneurisms as shown in Table 1 [12-20].
|
Reference |
Number of patients |
Previous treatment |
Reason for using Anakinra |
Doses and duration of Anakinra |
Outcome |
|
[12] |
1 |
1 IVIG 1 Steroid bolus |
Macrophage activation syndrome |
3 mg/kg/day 3 days than 8 mg/kg/day 5 months |
Normalization of previous abnormalities |
|
[13] |
1 |
1 IVIG |
Coronary aneurysms |
4 mg/kg/day 2 months |
Normalization of the Z score |
|
[14] |
1 |
2 IVIG, 3 Steroid boluses 1 Infliximab |
Giant aneurysms |
6 mg/kg/day 9 weeks |
Progressive clinical improvement, Normalization of inflammatory markers, Resolution of aneurysms |
|
[15] |
11 |
At least 2 IVIG |
Persistent fever, dilatation of coronary arteries, laboratory abnormalities myocarditis with KD shock syndrome |
Range 2–8 mg/kg/day & 6-81 days |
Resolution of fever, Reduction of inflammation indexes, and of the Z score |
|
[16] |
1 |
2 IVIG 4 Steroid boluses |
Persistent fever and biological abnormalities |
2 mg/kg/day 2 weeks |
Normalization of clinical, biological and echography abnormalities |
|
[17] |
1 |
2 IVIG 1 Infliximab 3 Steroid boluses |
Macrophage activation syndrome |
5 mg/kg/day 1 day then 10 mg/kg/day tapering in 6 months |
Gradual resolution of clinical symptoms, Normalization of laboratory tests |
|
[18] |
1 |
2 IVIG 3 Steroid boluses |
Worsening of coronary aneurysms |
6 mg/kg/day 10 weeks, Tapered to 6 mg/kg/every 2 days 4 weeks, tapered to 6 mg/kg/ every 3 days 4 weeks |
Apyrexia, Normalization of biological abnormalities, Reduction of aneurysms |
|
[19] |
1 |
1 IVIG |
Coronary aneurysms and increased proBNP levels |
100 mg/day 4 weeks |
Resolution of clinical and biological abnormalities, Disappearance of the stenosis and persistence of slight dilatation only in the proximal portion of the main vessels |
|
[20] |
16 |
1 or more IVIG |
Persistent fever, clinical and biological abnormalities |
2mg/kg/day 4mg/kg in patients ≤ 8 months and ≥ 5 kg, increased up to 6mg/kg/d if temperature remained >38°c 14 days |
Apyrexia, Reduction of disease activity, Normalization of CRP, Improvement of Z score |
Table 1: Case reports of of Anakinra use in resistant Kawasaki disease.
4. Conclusion
Our experience provides an additional argument for prescribing Anakinra as a second or third-line treatment in some cases of refractory Kawasaki disease, especially in cases of severe coronary artery involvement.
Authors’ Contribution
KB conceptualized, and critically reviewed the manuscript for important intellectual content. SL is the resident who drafted the manuscript. AS is a permanent pediatric rheumatologist in the unit. AD did the echocardiographic follow up. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing Interest
The authors declare that they have no conflict of interest.
References
- Mc Crindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135 (2017): e927-99.
- Burns JC, Capparelli EV, Brown JA, et al. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome study group. Pediatr Infect Dis J 17 (1998): 1144-1148.
- Tremoulet AH, Best BM, Song S, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr 153 (2008): 117-121.
- Dusser P and Koné-Paut I. IL-1 inhibition may have an important role in treating refractory Kawasaki disease. Front Pharmacol 8 (2017): 163.
- Lee Y, Schulte DJ, Shimada K, et al. Interleukin-1β is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 125 (2012): 1542-1550.
- Wallace CA, French JW, Kahn SJ, et al. Initial intravenous gammaglobulin treatment failure in kawasaki disease. Pediatrics 105 (2000): E78.
- Shrestha S, Wiener H, Shendre A, et al. Role of activating fcgR gene polymorphisms in kawasaki disease susceptibility and intravenous immunoglobulin response. Circ Cardiovasc Genet 5 (2012): 309-316.
- Burns JC, Newburger JW. Genetics insights into the pathogenesis of kawasaki disease. Circ Cardiovasc Genet 5 (2012): 277-278.
- Hoang LT, Shimizu C, Ling L, et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med 6 (2014): 541.
- Weng KP, Ho TY, Chiao YH, et al. Cytokine genetic polymorphisms and susceptibility to Kawasaki disease in Taiwanese children. Circ J 74 (2010): 2726-2733.
- Cohen S, Tacke CE, Straver B, et al. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis 71 (2012): 2059-2061.
- Shafferman A, Birmingham JD, Cron RQ. High-dose anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatr Rheumatol Online J 12 (2014): 26.
- Maggio MC, Cimaz R, Alaimo A, et al. Kawasaki disease triggered by parvovirus infection: an atypical case report of two siblings. J Med Case Rep 13 (2019): 104.
- Gambacorta A, Buonsenso D, De Rosa G, et al. Resolution of giant coronary aneurisms in a child with refractory Kawasaki disease treated with anakinra. Front Pediatr 8 (2020): 195.
- Koné-Paut I, Cimaz R, Herberg J, et al. The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: a retrospective cases series.Autoimmun Rev 17 (2018): 768-774.
- Sánchez-Manubens J, Gelman A, Franch N, et al. A child with resistant Kawasaki disease successfully treated with anakinra: a case report. BMC Pediatr 17 (2017): 102.
- Lind-Holst M, Hartling UB, Christensen AE. High-dose anakinra as treatment for macrophage activation syndrome caused by refractory Kawasaki disease in an infant. BMJ Case Rep 12 (2019): e229708.
- Guillaume MP, Reumaux H, Dubos F. Usefulness and safety of Anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol Young 28 (2018): 739-742.
- Blonz G, Lacroix S, Benbrik N, et al. Severe late-onset Kawasaki disease successfully treated with anakinra [published online 652 G. Ferrara et al. ahead of print, 2018 Jun 15]. J Clin Rheumatol (2018).
- Koné-Paut, I, Tellier S, Belot A, et al. Phase II Open Label Study of Anakinra in Intravenous Immunoglobulin–R


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
