A Severe Case of Invasive Rhino-Orbito-Cerebral Mucormycosis in a Patient with Acute T-Cell Leukemia with Successful Outcome: a Case Report and a Literature Review
Article Information
Linas Davainis1*, Dominyka Vasilevska2, Andrius Zucenka1,2, Birute Davainiene1, Regina Pileckyte1, Laimonas Griškvevicius1
1Hematology, Oncology and Transfusion Medicine Center, Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania
2Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
*Corresponding Author: Linas Davainis, Hematology, Oncology and Transfusion Medicine Center, Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania.
Received: 16 January 2023; Accepted: 24 January 2023; Published: 03 February 2023
Citation: Linas Davainis, Dominyka Vasilevska, Andrius Žučenka, Birutė Davainienė, Regina Pileckytė, Laimonas Griškvevičius. A Severe Case of Invasive Rhino-Orbito-Cerebral Mucormycosis in a Patient with Acute T-Cell Leukemia with Successful Outcome: a Case Report and a Literature Review. Archives of Clinical and Medical Case Reports 7 (2023): 58-65.
View / Download Pdf Share at FacebookAbstract
Mucormycosis is infective disease caused by fungi in order of Mucorales. The fungi invade blood vessels wall, causing infarction and necrosis that are difficult to be reached by antifungal agents, resulting in challenging treatment. Mucormycosis usually occurs in severelly immunosupresed patient, complicating treatment of hematological malignancies and increasing mortality, despite successful treatment of the oncological disease. Mucormycosis carries very high mortality rate (50-85%), some forms, like rhino-orbito-cerebral, and determines over 95% mortality. This manuscript presents a severe case of invasive rhino-orbito-cerebral mucormycosis in a patient after chemotherapy for acute T-cell leukemia with successful outcome after treatment with extensive surgical debridement concomitant with liposomal amphotericin B (LAmB) and isavuconazole, while simultaneously reducing immunosuppression. Despite successful outcome of mucormycosis and sustained leukemia remission rapid spread the invasive fungal infection led to permanent blindness.
Keywords
Acute T-Cell Leukemia; Bilateral Orbital Exenteration; Invasive Fungal Infection; Invasive Mucormycosis; Isavuconazole; Lichteimia Corymbifera; Liposomal Amphotericin B; Rhino-Orbito-Cerebral Mucormycosis
Acute T-Cell Leukemia articles; Bilateral Orbital Exenteration articles; Invasive Fungal Infection articles; Invasive Mucormycosis articles; Isavuconazole articles; Lichteimia Corymbifera articles; Liposomal Amphotericin B articles; Rhino-Orbito-Cerebral Mucormycosis articles
Article Details
1. Introduction
Invasive fungal infections (IFI) are one of leading cause mortality in immunocompromised patients with hematologic malignancies, even despite successful treatment of the oncological disease. The third most common cause of IFIs in immunocompromised patients (after invasive candidiasis and invasive aspergillosis) is mucormycosis, which is increasing in frequency, life-threatening, IFI caused by various species belonging to the subphylum Mucoromycotina, order Mucorales [1–3]. Mucormycosis carries very high mortality rate, some forms, like rhino-orbito-cerebral (ROC) mucormycosis determines over 95 % mortality. ROC mucormycosis is involved in between 33 % and 50 % of all cases of mucormycosis. Indicators of dismal prognosis include a postponing of treatment for more than 6 days, evidence of intracranial, bilateral or palate invasion, underlying hematological malignancy [4]. The principal feature of disease progression covers angioinvasion and tissue necrosis that leads to fungal dissemination through the blood stream, inducing deeper infections that are difficult to be reached by antifungal agents. Hence, antifungal therapy ought to be combined with surgical removal of the site [1,4]. Reliable data for the treatment of mucormycosis from prospective, randomized clinical studies is lacking, due to its‘ rare nature and limited awareness of the mold (despite recently surging cases), heterogeneity of hosts and sites of infection, multiple agents causing mucormycosis and challenging fungus obtainment from microbiological culture [5]. This manuscript presents a severe case of invasive mucormycosis (ROC form) in a patient after chemotherapy for acute T-cell leukemia with successful outcome after treatment with extensive surgical debridement concomitant with liposomal amphotericin B (LAmB) and isavuconazole. Despite successful outcome rapid spread and high pathogenicity of mucormycosis led to permanent visual loss.
2. Case Report
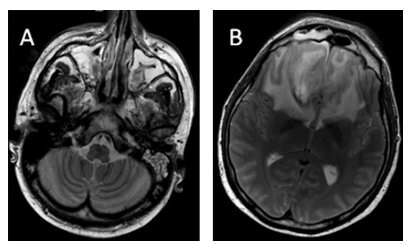
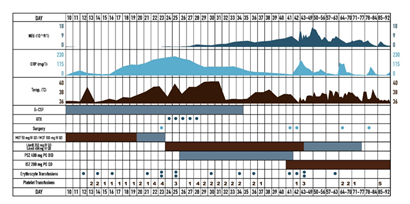
38 years old male patient sought medical advice due to painful neck nodule and dyspnea. After CT scan, superior mediastinum tumor, dislocating trachea and pathological left side neck lymph nodes were noted. Mediastinal biopsy was performed and the patient was diagnosed with acute T-cell (prothymocytic) leukemia / T-lymphoblastic lymphoma, nodal involvement and extranodal spreading, with Ki67 activity 80%. Bone marrow showed minimal abberant T-lymphoblastic infiltration (2,6 % aberrant T-lymphoblasts by flow cytometry). Complex karyotype (with 16q dup, 17p del, 13q del abberations) and clonal TCRD profile were founded. PET/CT investigation indicated right supraclavicular, left cervical and supraclavicular lymph nodes, upper mediastinal, retrosternal and upper intercostal masses uptake FDG, Lugano IIE. Induction therapy according to NOPHO HR protocol was carried out until day 15. Patient were stratified into high risk group, because of T-lineage malignancy and insufficient response to induction chemotherapy. Intensive chemotherapy blocks were initiated. Patient undergone NOPHO HR A and B block with mild infectious complications. After completion of NOPHO HR BLOCK C chemotherapy (idarubicin, high dose cytarabine, fludarabine, pegylated asparaginase), negative CRP dynamic was observed and S. haemolyticus MR sepsis, probable CVC origin were diagnosed, and treated with linezolid 600 mg BID. The patient developed absolute neutropenia on day 10. During post-cytostatic neutropenia period patient received antifungal prophylaxis with AmB oral suspension and intravenous micafungin 50 mg QD, although G-CSF 5 mcg/kg/d was prescribed. On NOPHO HR C1 block day 20, the patient complained of severe headache and fever. Head CT scan showed no abnormalities, no focal neurological symptoms were observed, only photophobia was noted. Micafungin dose was increased to 100 mg QD. On day 21, the headache persisted, eyelid swelling, hyperlacrimation, nasal congestion was observed. CT scan showed slight thickening of sinus mucosa. Meropenem was suspected to be the etiology of headache, hence it was changed to ceftazidime. Headache control with morphine and carbamazepine was continued. On day 22, eyelid oedema was observed, repeated CT scan showed superior sagittal sinus thrombosis with frontal bilateral hemorrhage and local intracerebral hemorrhage in right frontal lobe. The patient was treated with osmotherapy and anticoagulation, keeping PLT levels above 50x10e9/l. Treatment with intravenous acyclovir was added, as HSV encephalitis was suspected. CRP persisted to rise in the agranulocytosis background. On day 23, periorbital hematoma in right medial angle of eyelid appeared, cerebral MRI scan was performed, suggesting the diagnosis of ROC mucormycosis (paranasal sinuses inflammatory infiltrative lesions with nose and ethmoidal mucosa necrosis; both frontal lobes oedema with right lobe subacute hemorrhage and multiple small hemorrhages bilaterally, suggesting septic cerebral vein thrombosis and venous infarcts) (Figure 1). Total endoscopic bilateral ethmoidotomy was performed, with concurrent initiation of treatment with systemic LAmB 350 mg (5 mg/kg) together with AmB eye drops QID. Lacking clinical improvement after LAmB oral suspension of posaconazole 400 mg BID were added. Due to severe and prolonged neutropenia, treatment with granulocyte transfusions was set up. Histopathological investigation showed invasive fungal sinusitis with severe angioinvasion, without significant inflammatory process (due to neutropenia), suggesting Mucor spp. infection, highlighting the need of microbiological verification to exclude mixed infection Culture from right nostril confirmed histopathological diagnosis, as Lichteimia corymbifera (Mucor) was grown. Susceptibility to AmB, posaconazole and marginal susceptibility to itraconazole was reported. On day 28, periorbital edema had progressed together with exophthalmos, necrotic lesions on dorsal part of the nose and bilaterally in medial angles of the eye orbits had appeared, although neutrophil counts showed positive dynamics (Figure 2). On the following day, incision and decompression of right orbit was performed, no discharge was obtained, although deep necrotic lesions were observed. Granulocyte transfusions stopped after 5 transfusions due to signs of granulopoiesis recovery on day 29. Cerebral MRI scan showed progressing frontal oedema, although there were no indications for neurosurgery. Until day 35, headache had regressed, although patient became disorientated, necrotic lesions had spread, patient became blind with right eye. Antibiotic therapy was upgraded to tigecycline and rifaximin due to A. baumanii growth. From day 38, considerable negative clinical and laboratorial dynamic was observed (worsening sight of left eye, chemosis, necrotic lesions progression), with progressive disease in sinuses, orbits and brain on repeated MRI scans on day 41, indicating the need of urgent debridement surgery (Figure 3). Based on better in vitro susceptibility (posaconazole 1 mg/l vs isavuconazole 0,38 mg/l), posaconazole was changed to isavuconazole 200 mg TID for 2 days and following 200 mg QD. This shift was also influenced by the fact that only posaconazole suspension was available, which was complicated in administration due to multiple interactions with food and other drugs. On day 41, because of intracranial hypertension progression and deteriorating level of consciousness, bifrontal craniotomy with frontal lobe mucormycosis abscess and granuloma removal was performed. Histopathological investigation of the abscess confirmed fungal etiology, L. corymbifera was grown from cerebral abscess culture. On day 42, further surgical debridement was performed, with bilateral orbital exenteration and tracheostomy formation: removal of nasal bones, medial walls of the orbits, nasal septa, ethmoidal cells, with sphenoidal and maxillary sinuses opening for necrotic mass debridement (Figure 4). Same antifungal (although LAmB daily dose was increased to 450 mg (7 mg/kg) and antibacterial treatment described earlier was continued in ICU, where patient became hemodynamically stable with adequate spontaneous breathing through tracheostomy tube and satisfactory communication. Surgery wounds were considerably colonized with MDR bacterias (multidrug-resistant A.baumanni, E.kobei, E.faecium), without systemic manifestation, hence the wounds dressing were moistened with AmB and tigecycline. Inflammatory markers decreased. Control MRI scan showed nonhomogeneous mases in the location of the previous surgery, but overall radiological investigation results had improved with no need for additional neurosurgical intervention. Although face wounds were contaminated with multidrug resistant microorganisms, and served as temporary infectious source, decision was made to perform eye orbits and sinuses reconstructive surgery 24 days after exenteration surgery, covering the face skin defect with the transverse double loop technique using latissimus dorsi myocutaneous flap. Despite absence of aseptic surgical field, no major complications occurred during postoperative period, flap vitality was adequate, only meropenem was added due to increasing inflammatory markers. However, one week after reconstructive surgery, necrosis developed on the borders of the flap, reconstructive surgery was repeated, using scalp skin (Figure 5). Afterwards, no fungus was found cytologically or in the culture from the wound discharge, inflammatory markers stabilized, antibiotic therapy was gradually discontinued together with LAmB, continuing only isavuconazole 200 mg p/os for 9 months. In control bone marrow biopsy, complete T-ALL remission was found, MRD was undetected by flow cytometry and clonal TCRD clonality PCR. Patient continues maintenance therapy (6-MP and MTX), ECOG – 0, subtle cognitive and emotion deficit developed. The patient was discharged from the hospital 70 days after the diagnosis of rhino-orbito-cerebral mucormycosis and 92 days after hospitalization. One year after the mucormycosis diagnosis, the patient is still treated with 6-MP and MTX, lumbar punctions every 12 weeks (no fungus growth in cerebrospinal fluid). No mucormycosis relapse was observed after 9 months of isavuconazole maintenance treatment. Patient’s clinical and laboratorial parameters and main treatment highlights are presented in Figure 6.
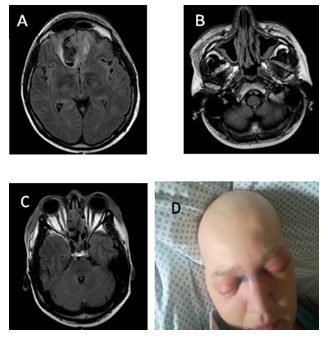
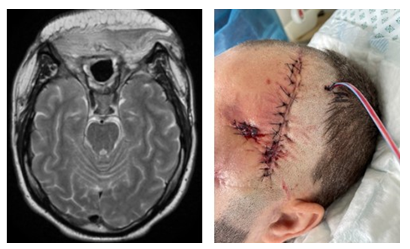
Figure 1: MRI scan and clinical presentation on day 23. A) MRI scan: Oedema of both frontal lobes. Subacute right frontal hematoma (venous stroke) due to thrombosis of superior sagittal sinus, oedema of frontal sinus mucosa. B) MRI scan: Paranasal sinuses (maxillary), nasal turbinates and facial soft tissue infiltration/inflamation. C) MRI scan: Ethmoidal cells infiltration, fluid acumulation and focal bone destruction. D) Clinical presentation: Periorbital hematoma in right medial angle of eyelid.
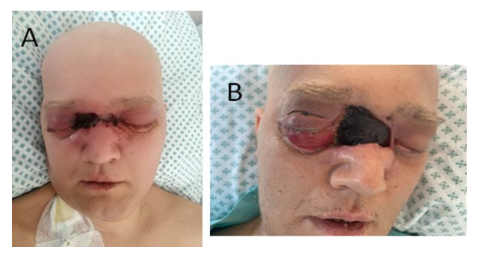
Figure 2: Clinical presentation. A) Clinical presentation on day 26: extension of necrotic eschar on dorsal part of the nose and bilaterally in medial angles of the eye orbits. B) Clinical presentation on day 30: Periorbital edema, exophthalmos, eschar extension with superficial skin necrosis on dorsal part of the nose and bilaterally in medial angles of the eye orbits, marked chemosis and right lower and upper eyelid incisions.
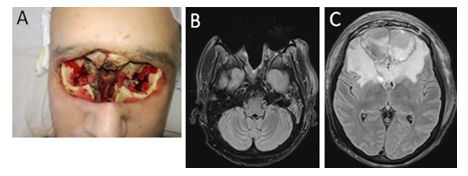
Figure 4: MRI scans and clinical presentation on day 45: A) Clinical presentation: view after bilateral orbital exenteration, nasal and ethmoidal bones removal, massive facial soft tissue defect and unclear residual bone viability. B) MRI scan: view after bilateral orbital exenteration, nasal and ethmoidal bones removal, massive facial soft tissue defect. C) MRI scan: view after bilateral frontal lobe abscess / granuloma evacuation, residual cerebral defect filled with heterogenous fluid, perifocal oedema, frontal sinuses reduction.
3. Discussion
This case represents a severe case of ROC mucormycosis (caused by L. corymbifera) in a patient with T-cell leukemia after intensive chemotherapy and prolonged neutropenia. Few years ago, updates on the taxonomy of Mucorales emphasizing clinically important taxa was performed. Istorically, Lichtheimia was classified under Absidia genus. Recently, evidence showed that Lichtheimia is distinct thermotolerant specie, varying in virulence and biological aspects [6]. Lichtheimia spp. in Europe is second most frequent agent of mucormycosis after Rhizopus spp. [6,7]. The most common underlying condition for Lichtheimia is hematological malignancy and prolonged severe neutropenia, though rhino-cerebral form is rare [6]. In developed countries mucormycosis remains mostly seen in patients with hematological malignancies, undergoing chemotherapy or allogeneic stem cell transplants [2]. Patients receiving antifungal prophylaxis with either itraconazole or voriconazole may be at increased risk of disseminated mucormycosis. Evidence exists of breakthrough mucormycosis in patients receiving posaconazole or echinocandin prophylaxis [8]. The patient described in this case received micafungin prophylaxis, which may have acted as a catalyst for invasive disease. From few available case reports, evidence exists that micafungin has no activity against molds of the order Mucorales with breakthrough infections within one week of starting micafungin. The routine use of voriconazole prophylaxis in many hemato-oncological centers has been controversially linked to an increase in mucormycosis cases, thus mucormycosis should be strongly suspected in any heavily immunosuppressed patient on voriconazole prophylaxis who develops fulminant sinusitis or nodular lung lesions [4,9–11]. The successful treatment of mucormycosis requires early diagnosis (clinical and laboratory), reversal of underlying predisposing risk factors and immunosuppression if possible, surgical debridement of necrotic lesions and timely antifungal therapy with possible adjunctive treatment [12]. Early diagnosis and prompt therapeutic intervention is critical, as in a study of patients with hematological malignancies and mucormycosis, delayed antifungal therapy for ≥6 days after diagnosis resulted in a 2-fold increase in mortality rate [13]. Timely surgical debridement, repeated on demand, is considered a key aspect of favorable outcome [14]. As in our case, with extensive spread of the disease, orbital exenteration, along with removal of the sinuses, may be necessary [15]. Local control was obtained in 90% of the patients after radical surgery vs. 41.6% in patients who had limited surgery. a 10 year retrospective study of 44 patients with hematologic malignancy and invasive fungal sinusitis, caused by mucormycosis agents in 13 cases, showed that early endoscopic approach did not improve overall outcome. The major risk factors for death, not fungal-related, were relapsed and/or refractory malignancy and protracted neutropenia [13]. Some other authors also agree, that the role of routine orbital exenteration or the timing of exenteration is currently unclear, and cases with orbital involvement have also been successfully managed without exenteration, putting emphasis on using less disfiguring surgical procedures [12]. Hence, the prognosis of our patient was dismal, since he had significant underlying neutropenia, hematological malignancy, disseminated disease with brain involvement. It could be argued about the diagnostic delay, since severe headache and photophobia appeared on day 20, while mucormycosis was suspected on head MRI scan on day 23, although early unspecific signs of mucormycosis such as fever and orbital pain could be dated back to day 14-15. In this case, the diagnosis was particularly based on clinical symptoms and imaging, as it took 2 weeks to culture the fungus (first nasal swab was even unsuccessful). In addition, patient’s leukemia was well controlled in addition to successful reversion of neutropenia with granulocyte transfusions and G-CSF. It could be argued that surgical debridement and orbital exenteration was performed with delay, 18 days after suspicion of mucormycosis on head MRI scan. However, the debridement was extremely radical, possibly leading to favorable outcome and first surgical intervention (total endoscopic bilateral ethmoidotomy) was performed as soon as mucormycosis was suspected on head MRI. In our tertiary care institution, 3 more mucormycosis cases occurred. Interestingly, in 3 out of 4 cases mucormycosis developed in patients with T-cell acute leukemia, the fourth case occurred in a patient with diffuse large B-cell lymphoma. First patient developed sinonasal mucormycosis after NOPHO HR Block A. Ethmoidotomy, antrostomy and sphenoidotomy extra was performed, prescribing antifungal treatment with micafungin 150 mg IV QD, later with Voriconazole 400 mg IV BID and Posaconazole 200 mg PO QID. The patient did not reached remission after chemotherapy (stable disease), died 7 days after suspicion of diagnosis due to carotid artery dissection. Another patient, with progressive DLBCL developed oral cavity and sinonasal mucormycosis due to Mucor circinelloides after R-CHOP. Surgical treatment was not performed, the patient was treated with voriconazole 200 mg p/os BID with topical AmB 20 mg TID and died 8 days after suspicion of diagnosis due to palatal perforation. The third case of mucormycosis, pulmonary form caused by Rhizopus microsporus, occurred in the patient with stable T-cell leukemia after NOPHO HR Block A. The patient was treated with lung S6 segmentectomy, AmB 100 mg IV QD with posaconazole 400 mg p/os BID and 6 granulocyte transfusions, followed by maintenance treatment with posaconazole 400 mg p/os BID. The patient died 6 months after diagnosis due to progression of leukemia. Our center experience suggests that the main factors influencing favorable outcomes are reversion of immunosuppression and myelotoxicity (mainly with adjuvant treatment, such as granulocyte transfusions), proper antifungal treatment (systemic polyene combined with III generation azole), radical surgical treatment, proper control of local complications and remission of hematological disease. According to the evidence available, the first clinical signs in ROC mucormycosis are nonspecific and include headache, facial swelling and pain, periorbital edema, nasal congestion and discharge, postnasal drip, dark blood-tinged or purulent rhinorrhea, sinus tenderness, fever, malaise, epistaxis, facial pain, ophtalmoplegia, chemosis, proptosis, ptosis [8,16,17]. The time from onset of early symptoms to late symptoms (necrotic ulcers, loss of vision, cranial nerve palsies, altered mental state) and signs that are diagnostic of the disease may be as short as one day, although in our case it took 8 days for necrotic lesions to progress from the onset of headache. The development of late symptoms and signs indicates a poor prognosis [15,18]. Black sores on the palate or nasal mucosa are very suggestive of mucormycosis in the appropriate clinical context, although they may not be present in 50% of cases [8]. Initial CT and MRI scans are sometimes unremarkable during the initial stages, but show evidence of rapid infection progression 2-3 days later. Therefore, serial radiographic imaging is important in patients with suspected mucormycosis, as was performed in the outlined case [19]. Histology/microscopy and culture still remain the gold standard despite recent advances in diagnostics [8,11,19]. Direct microscopical examination of sputum, sinus secretions, bronchoalveolar lavage fluid or sterile specimens is quick approach for a presumptive diagnosis, without identification of genus or species [2]. In patient with ROC mucormycosis, usually sinus biopsy is performed due to easy accessibility through nasal endoscopy [5]. Biopsy reveals characteristic wide, thick-walled, empty, irregular diameter, ribbon-like due to irregular branching, aseptate hyphal elements that branch at right angles variable up to 90° (wide-angle bifurcations) [2,20]. Other fungi, including Aspergillus, have septa, are thinner, and branch at acute angles. Even when hyphae are seen during microscopy, culture recovery is only positive in 50% of cases because of the inherently fragile nature of aseptate hyphae [20]. Molecular assays are valuable add-on tool that complement traditional diagnostic methods, leading to species-specific diagnosis. In addition, these tools can improve tissue diagnosis of culture-negative invasive mold infection. Molecular assays target the fungal ribosomal RNA gene (18S rRNA, 5.8S rRNA, 28S rRNA (including D1/D2), and 5S rRNA) as well as the internal transcribed spacers (ITS1 and ITS2,) and the intergenic sequences (IGS1 and IGS2). The use of the ITS region for species identification for Mucorales is widely accepted and supported by CLSI guidelines for resolution to genus and even species level [21,22]. However, it lacks discrimination of closely related species (sibling species). Thus, for proper differentiation of Lichtheimia spp. D1/D2 region is recommended [20,23]. Given the uncommon nature of infection and no direct evidence for prophylaxis directed solely towards mucormycosis, primary antifungal prophylaxis for mucormycosis is not recommended. In usual clinical setting prophylaxis is directed against a broad range of fungal infections, including candidiasis and aspergillosis [21]. As mentioned before, standard antifungal prophylaxis against over molds may even lead to breakthrough of resistant mucormycosis. However, breakthrough mucormycosis has been a rare event during prophylaxis with posaconazole oral suspension, and exposure due to posaconazole delayed release tablets, or intravenous infusions may result in even lower invasive fungal infection rates [18,21]. Isavuconazole is marginally supported in neutropenic patients and there is a lack of evidence to support liposomal AmB prophylaxis for patients receiving immunosuppressive treatment [24]. Instead of primary antifungal prophylaxis, the most important strategy for prevention of ROCM consists of maintaining adequate host defenses [5]. While Aspergillus species are typically susceptible to voriconazole, AmB, and echinocandins, Mucorales are usually susceptible only to AmB and less frequently to posaconazole and isavuconazole [24,25]. Mucorales species are inherently resistant to many antifungal agents, including fluconazole, voriconazole, flucytosine, caspofungin, 5-fluorocytosine, micafungin [21]. Newer triazoles, namely posaconazole and isavuconazole, show in vitro activity against Mucorales and clinical data supporting their use in mucormycosis [13]. Evidence from a considerable number of in vitro studies suggests that itraconazole, isavuconazole and posaconazole activity is highest against L. corymbifera, and lowest against Mucor spp. [26]. This may partially explain successful outcome in the reported case. In addition, evidence exists of posaconazole and isavuconazole being active in salvage therapy of patients with refractory mucormycosis or intolerant of AmB therapy [5]. New posaconazole formulations – intravenous and delayed release tablets (moderately recommended by the ECMM as first-line treatment) – were recently introduced, leading to better bioavailability than oral suspensions [24]. Isavuconazole is approved in the United States for the treatment of mucormycosis, and in Europe for the treatment of mucormycosis when AmB is not feasible. It has many pharmacokinetic benefits and less side effects compared to other azoles. Although posaconazole has lower MIC, isavuconazole reaches higher blood concentrations [24]. In the VITAL study conducted with patients with hematological malignancies, isavuconazole was used for primary treatment and complete or partial response was achieved in 32% of the patients by the end of therapy, and all-cause mortality was 43% after 3 months of treatment [27]. However, breakthrough mucormycosis and other IFIs cases in patients receiving isavuconazole as prophylaxis or treatment is a considerable issue. In the setting of deep immunosupression, suboptimal isavuconazole levels and prolonged administration selection of isavuconazole-resistant Mucorales may occur. Some authors suggest cross-tolerance in patients receiving extended posaconazole prophylaxis who develop breakthrough mucormucosis, which is resistant to following treatment with isavuconazole [13,24]. Evidence emerged, that isavuconazole may be effective in Mucorales CNS infection, supporting the treatment selection in the described case. Considerable penetration of this antifungal agent through blood-brain barrier into the inflammatory tissue around the abscess was demonstrated in animal model (24). First-line treatment with high-dose LAmB is strongly recommended both by The European Conference on Infections in Leukemia (ECIL) mucormycosis treatment guidelines in 2017 and the European Confederation of Medical Mycology (ECMM) update in 2019 and remains the only licensed antifungal agent for the treatment of mucormycosis [3,14,24]. Regarding the dosage of antifungal drugs, first-line treatment with liposomal AmB 5–10 mg/kg per day is strongly supported across all patterns of organ involvement. In the described case, the patient initially received 350 mg of LAmB (5 mg/kg per day), during the disease course the dosage was increased to 450 mg (7 mg/kg per day). The dose can be reduced as necessary (i.e. if renal toxicity develops), but doses below 5 mg/kg per day are recommended with marginal strength. The ECMM recommends 5–10 mg/kg and strongly 10 mg/kg in the event of CNS involvement [24,28]. LAmB at 5 mg ⁄ kg has not been shown to be inferior to higher doses [18]. Because of auto-induction of metabolism, which results in paradoxically lower drug levels, there is no advantage to escalating the LAmB dose above 10 mg/kg per day, and doses of 5 mg/kg per day are probably adequate for non-CNS infections [29,30]. Evidence suggests that AmB used for local instillation or irrigation of debrided cavities in surgical management leads to less need of exenteration [12,21,29,30]. Secondary antifungal prophylaxis should be considered for persistently immunosuppressed patients to prevent recurrence. Evidence exists of mucormycosis reactivation in patients with hematological malignancies following more than 2 years of continuous treatment and prior good clinical response to antifungal therapy [14]. Usually, continuing the last effective drug is recommended [21].The presented patient continues to take isavuconazole 8 months after being discharged from hospital, in the setting of leukemia remission and maintenance therapy (6-MP and MTX). Lack of standardized recommendations makes it challenging to guide further antifungal treatment length. Immune augmentation strategies, aiming in reversion of immunosuppression and myelotoxicity and including the administration of cytokines such as granulocyte (macrophage)-colony stimulating factor that boost phagocytic activity and oxidative respiratory burst and elevate neutrophil counts [4,8,11]. Granulocyte transfusions carry the risk of complications, including respiratory distress, alloimmunization, and anaphylaxis. On the other hand, granulocyte transfusions with cytokine augmentation may provide critical support to a neutropenic host with a life-threatening infection until recovery from neutropenia ensues, as happened in the presented case [5].
4. Conclusion
Although this case report presents successful outcome of a patient with invasive rhino-orbito-cerebral mucormycosis, patient ‘s treatment course from retrospective point raises questions about what could have been optimized in this case as well as in the future cases, primarily putting emphasis on the prevention of the development of invasive mucormycosis. First of all, the biggest challenge is in rising awareness of clinical suspicion of nonspecific symptoms, as early diagnosis is the core of favorable outcome. Antifungal prophylaxis remains a significant obstacle, as effective agents for one fungus may cause breakthrough infections of the other fungus. Development of molecular strategies towards rapid detection of the fungal infection agent and its susceptibility to antifungals are crucial to support standard time-consuming microbiological culture. Our experience suggests that control of underlying hematological malignancy and reversion of immunosuppression and myelotoxicity are key hallmarks of mucormycosis suppression. However, there is a lack of standardized approach, keeping in mind adverse effects of adjunctive treatment such as granulocyte transfusions. Even though there is no evidence supporting benefits of combination therapy, adding isavuconazole was probably rational due to CNS infection. Nonetheless, in addition to the uncertainty about the benefits of combining antifungals, question remains about the optimal LAmB doses on the individual bases and the duration of maintenance treatment. Surgical treatment causes significant wave of debates. While it is agreed to perform it without delay, doubts exist about the extent of debridement keeping in mind significant disfigurements after this approach.
Funding
This research received no external funding.
Disclosure of Interest
The authors report there are no competing interests to declare.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this report and the accompanying images.
References
- Beketova TR, Bailey L, Crowell EL, et al. Orbitocerebral Mucormycosis in a Patient with Central Nervous System Lymphoma. Ophthal Plast Reconstr Surg 34 (2018): e197-201.
- Lackner M, Caramalho R, Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol 9 (2014): 683-695.
- Spellberg B, Ibrahim AS. Mucormycosis. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine, 19e [Internet]. New York, NY: McGraw-Hill Education (2014).
- Sari ND, Serin I, Kirgezen T, et al. Mucormycosis in hematologic malignancies: Clinical follow-up and treatment results. Istanbul Medical Journal 22 (2021): 245-249.
- Gamaletsou MN, Sipsas NV, Roilides E, et al. Rhino-orbital-cerebral mucormycosis. Current Infectious Disease Reports 14 (2012): 423-434.
- Pan J, Tsui C, Li M, et al. First case of rhinocerebral mucormycosis caused by Lichtheimia ornata, with a review of Lichtheimia infections. Mycopathologia 185 (2020): 555-567.
- Walther, Wagner, Kurzai. Updates on the taxonomy of Mucorales with an emphasis on clinically important taxa. Journal of Fungi 5 (2019): 106.
- Abdollahi A, Shokohi T, Amirrajab N, et al. Clinical features, diagnosis, and outcomes of Rhino-Orbito-Cerebral mucormycosis: A retrospective analysis. Current Medical Mycology 2 (2016): 15-23.
- Bouza E, Muñoz P, Guinea J. Mucormycosis: An emerging disease? Clinical Microbiology and Infection 12 (2006): 7-23.
- Soare AY, Watkins TN, Bruno VM. Understanding Mucormycoses in the Age of “omics.” Front Genet 11 (2020): 699.
- Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: Guidelines from the 3rd european conference on infections in leukemia (ECIL 3). Haematologica 98 (2012): 492-504.
- AK AK, Gupta V. Rhino-orbital cerebral mucormycosis. [Updated 2022 Sep 19]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2022).
- Sipsas NV, Gamaletsou MN, Anastasopoulou A, et al. Therapy of Mucormycosis. J Fungi 4 (2018): 90.
- Ramadorai A, Ravi P, Narayanan V. Rhinocerebral Mucormycosis: A Prospective Analysis of an Effective Treatment Protocol. Ann Maxillofac Surg 9 (2019): 192-196.
- Murthy R, Gote YS, Bagchi A. Localized surgical debridement for the management of orbital mucormycosis. Indian J Ophthalmol 70 (2022): 649-652.
- Zayet S, Zaghdoudi A, Ammari L, et al. Cerebro-rhino-orbital mucormycosis and aspergillosis coinfection in a patient with diabetes mellitus: A case report. IDCases 23 (2021): e01022.
- Holmes TR, Hepschke JL, Jacobson I, et al. Mucormycosis: early treatment is the key to survival. Medical Journal of Australia 215 (2021): 401-403.
- Mohamed MS, Abdel-Motaleb HY, Mobarak FA. Management of rhino-orbital mucormycosis. Saudi Med J 36 (2015): 865-868.
- Asano-Mori Y. Diagnosis and Treatment of Mucormycosis in Patients with Hematological Malignancies [Translated Article]. Med Mycol J 58 (2017): e97-e105.
- Lackner N, Posch W, Lass-Flörl C. Microbiological and Molecular Diagnosis of Mucormycosis: From Old to New. Microorganisms 9 (2021): 1518.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19 (2019): e405-e421.
- Millon L, Scherer E, Rocchi S, et al. Molecular Strategies to Diagnose Mucormycosis. J Fungi 5 (2019): 24.
- Skiada A, Lass-Floerl C, Klimko N, et al. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol 56 (2018): S93-S101.
- Brunet K, Rammaert B. Mucormycosis treatment: Recommendations, latest advances, and perspectives. J Mycol Médicale 30 (2020): 101007.
- Bellazreg F, Hattab Z, Meksi S, et al. Outcome of mucormycosis after treatment: report of five cases. New Microbes New Infect 6 (2014): 49-52.
- Borman AM, Fraser M, Patterson Z, et al. In Vitro Antifungal Drug Resistance Profiles of Clinically Relevant Members of the Mucorales (Mucoromycota) Especially with the Newer Triazoles. J Fungi Basel Switz 7 (2021): 271.
- Miller MA, Molina KC, Gutman JA, et al. Mucormycosis in Hematopoietic Cell Transplant Recipients and in Patients With Hematological Malignancies in the Era of New Antifungal Agents. Open Forum Infect Dis 8 (2020): ofaa646.
- Garre V. Recent advances and future directions in the understanding of mucormycosis. Frontiers in Cellular and Infection Microbiology 12 (2022):
- Levinsen M, Kiilgaard JF, Thomsen C, et al. Medical and surgical treatment of rhino-orbital-cerebral mucormycosis in a child with leukemia. Am J Ophthalmol Case Rep 22 (2021): 101092.
- Baldin C, Ibrahim AS. Molecular mechanisms of mucormycosis—The bitter and the sweet. PLoS Pathog 13 (2017): e1006408.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
