Correlation between Proinflammatory Cytokines and Severity of COVID-19 within Palestinian Population
Article Information
Walid Basha1*, Zaher Nazzal1, Yousef El-Hamshary2, Anwar Odeh1, Lama Hijjawi1, Mahmoud Doden1, Ahmad Musa1, Saad Ruzzeh2
1Faculty of medicine and health sciences, An-Najah National University, Nablus, Palestine
2Ministry of Health, Nablus, Palestine
*Corresponding author: Walid Basha, Faculty of medicine and health sciences, An-Najah National University, Nablus, Palestine
Received: 25 May 2022; Accepted: 07 June 2022; Published: 22 June 2022
Citation: Walid Basha, Zaher Nazzal, Yousef El-Hamshary, Anwar Odeh, Lama Hijjawi, Mahmoud Doden, Ahmad Musa, Saad Ruzzeh. Correlation between Proinflammatory Cytokines and Severity of COVID-19 within Palestinian Population. Archives of Internal Medicine Research 5 (2022): 241-248.
View / Download Pdf Share at FacebookAbstract
COVID-19 was characterized by cytokine storm and endothelial dysfunction in severely ill patients. As the severity of the infection was correlated with ethnicity, this study aimed to assess the correlation between proinflammatory cytokine serum level and COVID-19 symptoms within the Palestinian population. In a cross-sectional study, serum samples of 27 non-hospitalized patients and 63 hospitalized patients SARS-CoV-2 infected patients were tested for total antibodies, IL-6, TNF-α, IFN-γ, and IL-1β using the ELISA test. Results showed that the most common symptoms within patients were joint pain, cough, and fever (73.3%, 69.7%, and 50%, respectively). Serum total antibodies (IGs) levels in non-hospitalized patients were higher than in hospitalized patients ((44.7 COI and 9.2 COI). TNF-α and IL-6 were lower in non-hospitalized patients than hospitalized patients (48 ± 17.9 pg/ml, 193.3 ± 350.5 pg/ml respectively). On the other hand, IFN-γ in non-hospitalized patients (1 ± 2 IU/ml) was significantly higher than hospitalized patients (0.4 ± 0.26 IU/ml). IL-1β was slightly lower in hospitalized patients (8.8 ± 13.6 pg/ml) compared to non-hospitalized patients (12.5 ± 24.5 pg/ml). Common mild symptoms of COVID-19 were negatively associated with proinflammatory cytokines serum level. In conclusion, as with other populations worldwide, IL-6 and TNF- α are playing a significant role in the complications of SARS-CoV-2 infection. Monitoring the two cytokines is crucial for the management and treatment of complicated consequences of COVID-19.
Keywords
COVID-19, Total Antibodies, Proinflammatory Cytokines, SARS-CoV-2
COVID-19 articles; Total Antibodies articles; Proinflammatory Cytokines articles; SARS-CoV-2 articles.
Article Details
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a worldwide emerging situation, initially reported in December 2019 in Wuhan, China, then affected countries worldwide increased, and it was declared a pandemic by the WHO [1]. The SARS-CoV-2 infection has a heterogenous disease course. It may be asymptomatic in the majority of the cases and can be mild to severe respiratory illness. However, more severe cases can be observed, such as multi-organ dysfunction syndromes, sepsis, and septic shock [2]. Morbidity and mortality in COVID-19 patients are accompanied by the secretion of an excessive storm of proinflammatory cytokines caused by the virus. Excessive production of proinflammatory cytokines is responsible for exacerbating acute respiratory distress syndrome and the extensive tissue damage responsible for multiple organ failures and death. Therefore targeting proinflammatory cytokines in severe complications in COVID-19 patients could increase patient survival rates and reduce mortality [3].
Transfusion of plasma from patients recovered from SARS-CoV-2 infection becomes an immuno-classical treatment for virus neutralization in severe COVID-19 cases. But for the shortness in plasma, the researchers created specific antibodies to neutralize virus particles simultaneously with modulating proinflammatory cytokines such as type I interferon, IL-6, and TNF-α that can reduce the severity of infection [4]. It has been shown that there is a correlation between the level of proinflammatory cytokines and ethnic groups. COVID-19 illness and hospitalization were varied among races due to genetic variation and chronic disease association [5]. This study investigates the correlation between cytokines level and specific antibody level with the symptom severity within the Palestinian population.
2. Material and Methods
2.1 Study design and participants
An observational cross-sectional study was conducted on COVID-19 positive patients. The study was conducted at the Martyrs medical military complex- Corona Hospital- Nablus, which is a government-run care facility for managing COVID-19 patients in the North of the West Bank of Palestine. Patients are known to have SARS-CoV-2 infection according to the Palestinian Ministry of Health (MOH) recommendations on SARS-CoV-2 diagnosis (RT-PCR). Ninety COVID-19 patients were enrolled in the study. Sixty-three of them were hospitalized COVID-19 cases with moderate or severe symptoms (Oxygen saturation was less than 92 and needed for supplemental Oxygen or has other organ failures) and categorized as the hospitalized patients (HP). Twenty-seven were asymptomatic or with mild symptoms and classified as non-hospitalized patients (NHP).
2.2 Variables and data collection
Patient demographic and clinical information, including signs and symptoms, chronic illnesses, and the date of potential exposure to the virus, were gathered from patient clinical records for hospitalized patients and direct interviews for asymptomatic non-hospitalized patients, after the patients or their relatives signed an agreement-consent form to use data in this study. Venous blood of about 5 ml blood sample was drawn in-plane tube from each study participant by a qualified lab technician and transported to the research laboratory at the faculty of Medicine and Health Sciences at An-Najah National University. Serum samples were kept at -80°C in sterile microtubes until the time of ELISA, following the manufacturer's instructions. Samples were taken from all patients before any anti-microbial or anti-inflammatory treatment administrations.
2.3 Total antibodies (IGs) and proinflammatory cytokines measurement
All serum samples were tested for IGs using the ELISA test (Elecsys® Anti-SARS-CoV-2 of the Roche Diagnostics Ltd). According to the manufacturer's recommendations. Results were considered positive above Cut of index 1 for total antibodies. In addition, proinflammatory cytokines serum concentration was tested by R&D system-Quantikine ELISA for IL-6 and TNF-α and DRG system ELISA for IFN-γ and IL-1β following the manufacture instructions.
2.4 Analysis and ethical consideration
All statistical analyses were done with IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY: IBM Corp). Continuous variables were expressed as mean ± standard deviation (SD). Counts and percentages described categorical variables. The Institutional Review Board (IRB) of An-Najah National University and the Ministry of Health Research Committee approved the study. Informed consent was obtained from each patient involved in this study or from a member of the patient family. No identifying data were collected during the study, and the data were to be only available to the research team.
3. Results
3.1 Background characteristics
The age of the study population ranged between 15 and 90 years old, with an average of 57.5 years old. Sample include 56 (62.2%) males and 31 (34.4%) females. The majority were residents in cities (62.2%) and non-smokers (84.4%). 73.3% suffered from chronic disease, 51% had diabetes, and 61% had hypertension. Previous yearly infection influenza comprised 32.2% of the sample, and only 7.8% received the annual influenza vaccine.
3.2 Symptoms associated with COVID-19
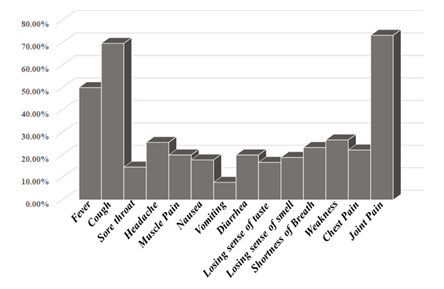
The symptoms of COVID-19 infection were varying in the incidence among patients. Joint pain was the most encountered symptom, constituting approximately 73.3%, followed by cough; 69.7%, and fever; 50 %. Other symptoms patients experience but at low incidence were weakness; 26.7%, headache; 25.6%, shortness of breath; 23.3%, chest pain; 22.2%, muscle pain; 20%, diarrhoea; 20%, nausea; 17.8%, losing sense of smell; 18.9%, losing sense of taste; 16.7% and sore throat; 14.6% (Figure 1).
3.3 Correlation between severity of COVID-19 and total IGs and cytokines serum level
Serum IGs levels in NHP (44.7 ± 56.6 COI) were significantly higher than HP (9.2 ± 14.7 COI), (p=0.012). TNF-α and IL-6 were significantly (p<0.001) lower in NHP compared to HP (48 ± 17.9 pg/ml, 193.3 ± 350.5 pg/ml respectively). In the contrary, IFN-γ, in NHP (1 ± 2 IU/ml) was significantly higher than HP (0.4 ± 0.26 IU/ml) (P=0.001). With no significant difference (p=0.827), IL-1β was slightly lower in HP (8.8 ± 13.6 pg/ml) compared to NHP (12.5 ± 24.5 pg/ml). (Table-1)
3.4 Correlation between Cytokine serum levels and common symptoms
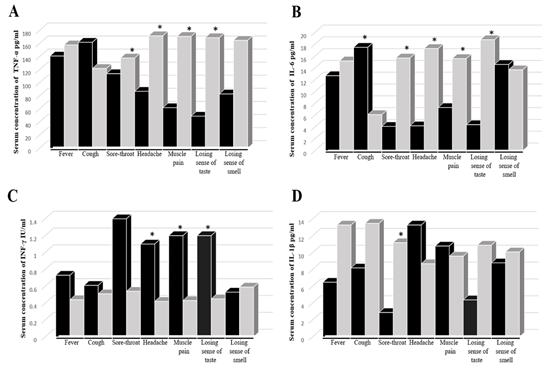
Cytokine's level did not show any difference in the absence or presence of nausea, vomiting, diarrhea, shortness of breath, weakness, chest pain, and joint pain. Meanwhile, the TNF-α concentration was significantly higher in the absence rather than the presence of sore throat, headache, muscle pain, loss of taste (Figure 2 A). It was similar with and IL-6 except for a significant elevation in the presence of cough (Figure 2 B). On the other hand, IFN-γ was higher in the present than in the absence of the symptoms, with significant elevation in the presence of headache, muscle pain, and loss of taste (Figure 2 C). Finally, the IL-1β level was significantly higher in the presence of headache and slightly with muscle pain and the absence of other symptoms (Figure 2 D).
|
Hospitalized (n=63) |
Non-Hospitalized (n=27) |
P-Value* |
|
|
Total Igs (COI) |
9.2 (±14.7) |
44.7 (±56.6) |
0.012 |
|
TNF-α (pg/ml) |
193.3 (±350.5) |
48.0 (±17.9) |
<0.001 |
|
IL-6 (pg/ml) |
18.2 (±34.6) |
4.2 (±0.88) |
<0.001 |
|
IFN-ϒ (IU/ml) |
0.4 (±0.26) |
1.0 (±2.0) |
0.001 |
|
IL-1β (pg/ml) |
8.8 (±13.6) |
12.5 (±24.5) |
0.827 |
Table 1: Total IGs, TNF-α, IL6, IFN-γ, IL-1β levels between hospitalized and non-hospitalized COVID-19 patients. *Mann-Whitney U test.
4. Discussion
Cytokine profiles and immune response in COVID-19 patients were deeply investigated in many countries worldwide due to the correlation between the severity of the disease and cytokine storm [6]. As in any other viral infection, proinflammatory cytokines and chemokines play an essential role in immune-pathology during viral infections, leading to hyperinflammatory responses in the pathogenesis of COVID-19 disease [7, 8]. However, the incident, severity, and complications of COVID-19 were varied between races and ethnic backgrounds [5, 9]. This variation refers to the differences in the immune repose and cytokine production between races [10, 11] which could be due to allelic distribution and genotype frequency, such as IL-2 and IL-6 [12].
Reports indicated the elevation of serum level of several proinflammatory cytokines, including IL-1β, TNF-α, IL-6, and T-cell cytokine IFN-γ in COVID-19 infection in general despite the severity of the disease [8, 13, 14]. The correlation with the severity of COVID-19 illness was confirmed with IL-6 [15, 16] but with variable results for other cytokines. This variation in results leads us to study the relationship of clinical symptom severity with the level of four cytokines within Palestinian COVID-19 patients. Our data showed a significant correlation between the severity of COVID-19 and the elevation level of IL-6 and TNF-α, with a nonsignificant elevation of IFN-γ, with no deference in IL-1β level between HP and NHP. Our study is in close corroboration with De Valle et al who suggested that IL-6 and TNF-α can be independent predictors of the disease severity [17]. This is, however, in contrast with Chen et al who demonstrated an increase in the IL-6 levels, but the concentration of TNF-α, IL-1, remained unaltered in severely affected patients [18]. The difference in TNF-α concentrations could be due to the race/ethnic background and the pathological condition, and the difference in sampling times. However, further research is needed for this topic.
The role of antibodies in the severity of COVID-19 is less clear. Normally, viral elimination requires cell-mediated immunity, but humoral immunity plays an essential role in eliminating viruses and infected cells such as antibody-dependent cell cytotoxicity (ADCC), opsonization and phagocytosis via innate immune cells. However, two COVID-19 cases of patients with X-linked gamma globulinemia were challenged with antibodies acquired and survived SARS-CoV-2 infection without severe complications [19]. Some studies have demonstrated and suggested a pathogenic role for antibodies in primary infection through enhancement and increased inflammation [20], although this is thought to be not enough to explain the prevalence of severe cases of infection [21]. As such, the beneficial, neutral, or harmful role of antibodies in active coronavirus infection remains controversial. Our results didn't show any correlation with the severity of the infection.
On the contrary with the severity of COVID-19, our results showed that IL-6 and TNF-α were neutral or negatively associated with the common symptoms except for cough, and IL-6, which was associated with losing sense of smell. Previous results suggested a significant role of IL-6 and TNF-α smell and taste dysfunction [22, 23]. This deference may be due to the difference in the population where those studies compared serum level of cytokines and chemokines in correlation with SARC-CoV-2 infection with healthy subjects, no correlation with the severity of the disease. This study has some limitations, including a limited sample size and a lack of time to follow up on patient changes. These factors may have played a role in the lack of a significant association of some of our findings, however they do not change the absence of any correlation between specific clinical severe symptoms and cytokine levels.
5. Conclusion
IL-6 and TNF-α have been playing a major role in the complications and severity of SARS-CoV-2 infection within Palestinians like other populations worldwide. But the difference was regarding the relationship of cytokines and common symptoms of infection. Therefore, monitoring IL-6 and TNF-α serum levels during disease stages is critical to managing and treating complicated consequences of COVID-19.
Acknowledgment
We thank the medical staff at Martyrs medical military
complex- Corona Hospital- Nablus for assistance with blood sampling and data collection, and laboratory technicians at the research lab center at An-Najah National University for their support in lab work. An-Najah National University funded this work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [W. Basha], upon reasonable request. A preprint of this article has previously been published [24].
Conflict of Interest
Walid Basha, Zaher Nazzal, Yousef El-Hamshary, Anwar Odeh, Lama Hijjawi, Mahmoud Doden, Ahmad Musa, and Saad Ruzzeh declare that they have no conflict of interest. Walid Basha as team leader has received research grants from the An-Najah National University for research development grant.
References
- Lai C-C, Shih T-P, Ko W-C, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents 55 (2020): 105924.
- Aminjafari A, Ghasemi S, Robson B, et al. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect , the company ’ s public news and information . Brain Behav Immun., S0889-1591 (2020).
- Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 Cytokine Storm; What We Know So Far. Frontiers in Immunology (2020).
- Shah V K, Firmal P, Alam A, et al. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Frontiers in Immunology (2020).
- Kopel J, Perisetti A, Roghani A, et al. Racial and Gender-Based Differences in COVID-19. Frontiers in Public Health, 8 (2020): 1-8.
- Akbari H, Tabrizi R, Lankarani K B, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Life Sciences 258 (2020): 118167.
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology 39 (2017): 529-539.
- Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 71 (2020): 762-768.
- Debnath M, Banerjee M, Berk M. Genetic gateways to COVID-19 infection: Implications for risk, severity, and FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 34 (2020): 8787-8795.
- A Haralambieva I H, Ovsyannikova I G, Kennedy R B, et al. Race and sex-based differences in cytokine immune responses to smallpox vaccine in healthy individuals. Human Immunology 74 (2013): 1263-1266.
- Schirmer M, Kumar V, Netea M G, et al. The causes and consequences of variation in human cytokine production in health. Current Opinion in Immunology 54 (2018): 50-58.
- Cox E D, Hoffmann S C, DiMercurio B S, et al. Cytokine polymorphic analyses indicate ethnic differences in the allelic distribution of interleukin-2 and interleukin-6. Transplantation 72 (2001): 720-726.
- He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID-19 pneumonia. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology, 127 (2020): 104361.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 395 (2020): 497-506.
- Broman N, Rantasärkkä K, Feuth T, et al. IL-6 and other biomarkers as predictors of severity in COVID-19. Annals of Medicine 53 (2021): 410-412.
- Galván-Román J M, Rodríguez-García S C, Roy-Vallejo E, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. The Journal of Allergy and Clinical Immunology 147 (2021): 72-80.
- Del Valle D M, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine 26 (2020): 1636-1643.
- Chen L, Liu H G, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia (2020).
- Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatric Allergy and Immunology: Official Publication of the European Society of Pediatric Allergy and Immunology 31 (2020): 565-569.
- Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight (2019).
- Arvin A M, Fink K, Schmid M A, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 584 (2020): 353-363.
- Cazzolla A P, Lovero R, Lo Muzio L, et al. Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chemical Neuroscience 11 (2020): 2774-2781.
- Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced ACS Chemical Neuroscience 11 (2020): 1909-1913.
- Basha W, Nazzal Z, El-Hamshary Y, et al. Correlation between Proinflammatory cytokines and severity of COVID-19 within Palestinian Population. MedRxiv (2021).


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 