Effect of Andrographis paniculata Treatment for Nonimmune Patients with Early-Stage COVID-19 on the Prevention of Pneumonia: A Retrospective Cohort Study
Article Information
Amporn Benjaponpitaka, Thiti Sawaengthama, Tewan Thaneerata, Kulthanit Wanaratnaa, Palang Chotsirib, Chalermquan Rungsawangc, Sakkarin Bhubhanilc, Sataporn Charoensukc, Suwat Benjaponpitakc, Sarawut Lapmaneec, Sayomporn Sirinavind*
a Department of Thai Traditional and Alternative Medicine, Ministry of Public Health, Nonthaburi, Thailand
b Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
c Department of Basic Medical Sciences, Faculty of Medicine, Siam University, Bangkok, Thailand
d Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand
*Corresponding Author: Sayomporn Sirinavin, MD, Division of Infectious Diseases, Department of Pediatrics
Faculty of Medicine Ramathibodi Hospital, Mahidol University. 270 Rama VI Road, Rajathevi, Bangkok 10400, Thailand.
Received: 19 May 2023; Accepted: 23 May 2023; Published: 31 May 2023
Citation: Amporn Benjaponpitak, Thiti Sawaengtham, Tewan Thaneerat, Kulthanit Wanaratna, Palang Chotsiri, Chalermquan Rungsawang, Sakkarin Bhubhanil, Sataporn Charoensuk, Suwat Benjaponpitak, Sarawut Lapmanee, Sayomporn Sirinavin. Effect of Andrographis paniculata treatment for nonimmune patients with early-stage COVID-19 on the prevention of pneumonia: A retrospective cohort study. Archives of Internal Medicine Research 6 (2023): 35-43
View / Download Pdf Share at FacebookAbstract
Background:
Andrographis paniculata (AP) is an herbal plant that has been used to treat upper respiratory tract infections. Andrographolide is the major active component of AP that inhibits intracellular SARS-CoV-2 replication and has anti-inflammatory action.
Objective:
To investigate the therapeutic and adverse effects of treatment with oral AP-products in patients with early-stage COVID-19.
Methods:
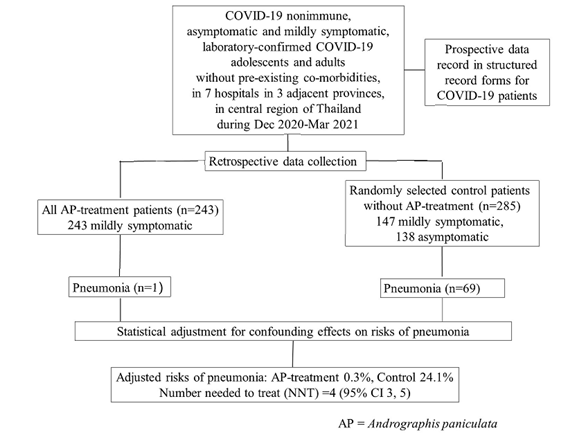
We performed a retrospective cohort study in COVID-19 patients with asymptomatic or mild COVID-19, admitted for isolation and treatment in seven hospitals in three adjacent provinces in central region of Thailand, during December 2020 – March 2021 epidemic when COVID-19 vaccine was not yet available and none were previously infected with SARS-CoV-2. Patient data was prospectively recorded in the structured medical record forms and retrospectively reviewed. This study included patients 15 to 60 years of age with laboratory-confirmed SARS-CoV-2 infection, but without comorbidities or pregnancy. Study AP products were capsules containing a standardised ethanol extract of AP or crude AP powder. Patients were treated for five days with either AP-extract (60 mg andrographolide, 3 times daily) or crude-AP (48 mg andrographolide, 3 times daily), only when available. All eligible patients who received AP treatment were included and control patients who did not receive AP treatment were blindly and randomly selected using a ratio of approximately 1:1. The risk of pneumonia diagnosed by chest radiography was the primary study outcome.
Results:
About 90% of the treatment group received the AP-extract regimen within 7 days after onset of symptoms. Pneumonia occurred in 1/243 AP treatment patients and 69/285 control patients. The risks of pneumonia after adjusting for confounding effects were 0.3% (95%CI, 0%-0.9%) and 24.3% (95%CI, 19.0%-29.7%) in the AP treatment and control groups, respectively. The number needed to treat to avoid pneumonia development in one patient was four (95% CI, 3-5). Mild abnormal symptoms suggesting adverse event of AP treatment were detected in eight patients.
Conclusion:
The oral AP-extract treatment regimen is acceptably safe and associated with highly reduced rates of pneumonia in nonimmune patients with early-stage COVID-19.
Keywords
Andrographis paniculata, andrographolide, pneumonia, SARSCoV- 2, COVID-19 treatment, COVID-19 nonimmune
Article Details
Abbreviations
Abbreviations: AP: Andrographis paniculata; COVID-19: Coronavirus Disease 2019; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; RT-PCR: Reverse Transcriptase Polymerase Chain Reaction
1. Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in December 2019, and it resulted in a global pandemic causing substantial global morbidity and mortality[1]. The widely affordable and easily accessible interventions to minimise losses caused by COVID-19 are needed. Patients with early-stage COVID-19 are asymptomatic or have mild symptoms. Patients with moderate COVID-19 commonly experience pneumonia development; these patients require hospitalisation or admission to an intensive care facility. Furthermore, some can experience respiratory failure, which may result in chronic lung diseases or death. Older age, male sex, and comorbidities have been associated with worse COVID-19 outcomes[2][3]. Early during its clinical course, COVID-19 is primarily driven by the intracellular replication of SARS-CoV-2 and upper respiratory tract mucosal cell damage. Later in its clinical course, COVID-19 appears to be driven by lower respiratory tract invasion and inflammatory responses to SARS-CoV-2, thus leading to pneumonia and organ tissue damage[4]. Patients with early-stage COVID-19 are generally self-limited but could progress to more severe diseases.
Andrographis paniculata (Burm.f.) Nees (AP) is an annual herb native to the subcontinent of India that has been widely introduced, naturalised, and cultivated in the tropical and subtropical regions of Asia[5]. This bitter-tasting herb is commonly called green chiretta; however, it has many local names, such as Kalmegh in India, Chuan Xin Lian in China, Sambiloto in Indonesia, Hempedu Bumi in Malaysia, and Fa Thalai Chon in Thailand. AP comprises several components that are known for their anti-microbial, anti-inflammatory, immunomodulation, anti-oxidant, and vasodilatation effects[6][7]. Oral AP has been used for centuries as a traditional medicine in India, China, Thailand, and other Asian countries. Several double-blind studies have demonstrated the benefits of AP for upper respiratory tract infections[8][9]. Andrographolide, which is a bitter bioactive diterpenoid, is the major active component of AP.
In silico or computer-based modelling studies have revealed that andrographolide from AP inhibits the main protease of SARS-CoV-2, which is an essential enzyme for viral replication, by successfully docking in its binding site[10][11][12][13][14]. An in vitro study of human lung epithelial cells demonstrated that AP extract and andrographolide treatment of cells infected with SARS-CoV-2 inhibited intracellular viral replication[15]. Furthermore, in silico and in vitro studies indicated that the anti-inflammatory effects of AP bioactive ingredients were related to reductions in the expression of interleukin-6 and tumour necrosis factor-alpha, which regulate inflammation and the immune response[16].
To optimise the dose, the pharmacokinetic parameters of the active components of AP were studied after multiple oral doses were administered to healthy Thai volunteers[17]. Dosage regimens to treat COVID-19 were proposed based on the results of clinical studies published in scientific literature and the anti-SARS-CoV-2 activity of AP extract in tissue culture[18]. Preliminary clinical experience with a small group of Thai patients suggested that AP treatment regimens could be effective for mild COVID-19[19].
AP is included in The National List of Essential Herbal Drugs A.D. 1999 of Thailand as an herbal drug used for the treatment of common cold symptoms and non-infectious diarrhoea. Two AP products were used as an emergency treatment for early-stage COVID-19 during the second wave of the COVID-19 epidemic in Thailand. We investigated the therapeutic and adverse effects of treatment with oral AP-products in COVID-19 nonimmune patients with early-stage COVID-19.
2. Patients and Methods
2.1 Study setting
The second wave of the COVID-19 epidemic in Thailand started in mid-December 2020 in fresh markets in Samut Sakhon Province[20]. It spread rapidly to the adjacent provinces while COVID-19 vaccines were not yet available and none were previously infected. Management of the spread of SARS-CoV-2 began at the onset of the outbreak. Molecular testing of the nasopharyngeal swabs or saliva samples using the reverse-transcriptase polymerase chain reaction (RT-PCR) to determine the presence of SARS-CoV-2 was performed to find cases in the community and patients who presented to the hospitals. Patients with laboratory-confirmed SARS-CoV-2 were promptly admitted to local hospitals, which were connected as networked medical care facilities, for isolation and treatment. Chest radiography was routinely performed at the time of admission.
All patients with asymptomatic or mild COVID-19 received general supportive care, including rest, hydration, vital sign monitoring, oxygen saturation monitoring, symptom treatment, and measures to prevent virus spread. The patients received AP treatment when it was available. Patients with more severe disease were treated separately. Patient data at the time of admission and during follow-up were regularly recorded in the structured medical records of COVID-19 patients by medical and paramedical personnel. Those who recovered were discharged from the hospital by day 14 of hospitalisation and followed up for at least 10 days. Those whose condition worsened were moved to medical care facilities for patients with more severe disease. The diagnoses of all patients at the time of discharge were recorded in the structured electronic medical records.
2.2 Study design
The retrospective cohort study was performed, using prospectively collected data from patients with asymptomatic or mild COVID-19 admitted in hospitals for isolation and treatment, during the second epidemic wave in Thailand. We compared the risks of pneumonia development for eligible patients who received AP treatment and a group of control patients who did not receive AP treatment.
2.3 Study medication
The active component of the study drug was andrographolide, which was obtained from AP. AP is a eudicot species in the Acanthaceae family (taxonomy ID: NCBI 175694) [21]. During the study period, two AP products were occasionally available as COVID-19 treatments in the included provinces. These were orally administered as capsules containing a standardised ethanol extract of the aerial part of AP (20 mg of andrographolide per capsule; AP extract) or standardised crude AP powder containing 400 mg of dried AP powder with 3% andrographolide (12 mg of andrographolide per capsule; crude AP). The AP extract was produced by Thai Herbal Products Co., Ltd. (Ayutthaya, Thailand), and crude AP was produced by the Chao Phya Abhaibhubejhr Hospital Foundation (Prachin Buri, Thailand). Both are herbal products registered with the Thailand Food and Drug Administration (registration numbers G169/62 and G512/60, respectively). The andrographolide concentration of each AP product was determined using high-performance liquid chromatography. During the 5-day course of treatment, each patient received either AP extract (60 mg of andrographolide, 3 times daily) or crude AP (48 mg of andrographolide, 3 times daily). Treatment with an AP product was offered to the patients on the admission date. Shortages of these AP products were common; the opportunity to receive AP treatment was dependent on the availability of AP products.
2.4 Patients
The patient inclusion criteria were as follows: age 15 to 60 years; laboratory-confirmed SARS-CoV-2 diagnosed by RT-PCR; asymptomatic or mild COVID-19 on admission with no evidence of lower respiratory tract problems; and admission to one of seven hospitals for COVID-19 isolation and treatment in three adjacent provinces (Samut Sakhon, Ratchaburi, and Nakhon Pathom) between December 2020 and March 2021. We excluded pregnant or breast-feeding women, those with any comorbidity associated with worse COVID-19 outcomes, and those who received any anti-viral drugs, herbs, or products containing andrographolide before hospital admission. Although mild obesity (body mass index, 30-34.9 kg/m2) had been classified as a risk factor for worse COVID-19 outcomes, patients with mild obesity were included in this study. The AP treatment was identified by reviewing the pharmacy records. All eligible patients treated with the AP regimens were included in the study. The control group included eligible patients who did not receive AP treatment; they were blindly and randomly selected (ratio of approximately 1:1 with the AP treatment group) from a database of hospitalised COVID-19 patients by non-clinical staff without knowledge of the study protocol and clinical course or outcomes of the patients.
2.5 Outcomes
The outcomes of interest were the occurrence of pneumonia confirmed by chest radiography and any probable adverse reactions to AP treatment described in the structured medical records. According to the standard of care of the hospitals during the study period, all patients underwent chest radiography at the time of hospital admission, when they developed suspicious signs or symptoms of lower respiratory tract infection, and before the decision regarding hospital discharge was determined.
2.6 Data collection
Data collection was conducted from April to May 2021. A team of physicians reviewed and collected demographic, clinical, and outcome data from the structured medical records of the included COVID-19 patients. Patient data were anonymised, and informed consent was not required.
2.7 Statistical analysis
All analyses were performed using Stata version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.), and p<0.05 was considered statistically significant. Data were described as the mean and standard deviation or frequency (n and %) and compared between groups using Student’s t-test or the chi-square and Fisher’s exact test for continuous and categorical data, respectively. A multivariate analysis was performed to adjust for potential confounding effects on the risk of pneumonia in the AP treatment and control groups. The treatment effect model was applied after inverse probability-weighted regression adjustment. First, a treatment model was constructed using a logit model by fitting the treatment to the covariates (i.e., age 51-60 years; body mass index >25-35 kg/m2; and clinical symptoms, including fever, cough, nasal congestion or runny nose, myalgia, loss of smell, loss of taste, and diarrhoea). Second, the outcome model was constructed using Poisson regression by fitting the pneumonia outcome to the covariates. The risk of pneumonia, risk difference, and number needed to treat were estimated.
3. Results
A total of 528 patients were included, none had previous SARS-CoV-2 vaccination or infection. There were 243 patients in the AP treatment group and 285 patients in the control group (Table 1). The two study groups were comparable in terms of average age, sex, and body mass index; however, the age group distribution was slightly different (p=0.035). All 243 patients in the AP treatment group and 53.0% (151/285) in the control group were symptomatic when AP treatment was initiated. This was not unexpected because of the shortage of AP products and because AP is a well-known treatment for acute respiratory tract infections. The proportion of patients with each symptom, except for diarrhoea, was higher in the AP treatment group than that in the control group. In the AP treatment group, 88.1% (214/243) received AP extract and 11.9% (29/243) received crude AP. The AP treatment was started within a week after onset of symptoms in 93.8% of the patients, i.e., 0-3 days, 53.1% (129/243); 4-7 days, 40.7% (99/243); and 8-13 days, 6.2% (15/243).
|
Characteristics |
AP treatment (n=243) |
Control (n=285) |
p-value |
|
|
Age (years), mean ± SD |
37.7±11.1 |
37.0 ±11.2 |
0.528 |
|
|
Age group (years), n (%) |
||||
|
15-30* |
84 (34.6) |
79 (27.7) |
0.035 |
|
|
31-40 |
69 (28.4) |
80 (28.1) |
||
|
41-50 |
51 (21.0) |
90 (31.6) |
||
|
51-60 |
39 (16.0) |
36 (12.6) |
||
|
Male, n (%) |
94 (38.7) |
106 (37.2) |
0.725 |
|
|
Body mass index (kg/m2), mean ± SD |
23.7 ± 4.1 |
24.0 ± 4.2 |
0.544 |
|
|
Being symptomatic on admission, n (%) |
243 (100.0) |
151 (53.0) |
<0.001 |
|
|
Presenting symptoms, n (%) |
||||
|
Fever |
85 (35.0) |
43 (15.1) |
<0.001 |
|
|
Cough |
136 (56.0) |
80 (28.1) |
<0.001 |
|
|
Nasal congestion or runny nose |
69 (28.4) |
51 (17.9) |
0.004 |
|
|
Myalgia |
37 (15.2) |
16 (5.6) |
<0.001 |
|
|
Loss of smell |
81 (33.3) |
49 (17.2) |
<0.001 |
|
|
Loss of taste |
30 (12.3) |
12 (4.2) |
0.001 |
|
|
Diarrhea |
10 (4.1) |
13 (4.6) |
0.802 |
|
*Ten patients in the AP treatment and 23 patients in the control group were adolescent (15 to 20 years).
Table 1: Baseline characteristics of COVID-19 patients in the Andrographis paniculata (AP) treatment and control groups.
Pneumonia occurred in one patient in the AP treatment group and 69 patients in the control group. The AP treatment patient who developed pneumonia was a 36-year-old man who received the crude AP regimen at 11 days after symptom onset. The risk of pneumonia in the control group was similar for patients with asymptomatic and mild COVID-19 (p=0.340), and it progressively increased with older age (p=0.001) (Table 2). The unadjusted risk of pneumonia for each potential risk variable was explored using a univariate analysis (Table 3). The adjusted risk of pneumonia in the control group was 24.3% (95% confidence interval [CI], 19.0%-29.7%); and it was 0.3% (95% CI, 0%-0.9%) in the AP treatment group. Therefore, the number needed to treat to avoid pneumonia development in one patient was four (95% CI, 3-5) (Table 4). Abnormal symptoms suggesting adverse effects of AP- treatment were detected in eight patients (nausea, 3; diarrhoea, 2; dizziness, 1; tachycardia, 1; chest tightness, 1). No substantial or persistent adverse effects of the AP treatment were observed in the study patients.
|
Variables |
Pneumonia, n (%) |
p-value |
||
|
Yes |
No |
|||
|
Overall, n (%) |
69 (24.2) |
216 (75.8) |
||
|
Age group (years), n (%) |
||||
|
15-30 |
11 (13.9) |
68 (86.1) |
0.001 |
|
|
31-40 |
16 (20.0) |
64 (80.0) |
||
|
41-50 |
25 (27.8) |
65 (72.2) |
||
|
51-60 |
17 (47.2) |
19 (52.8) |
||
|
Clinical classification on admission, n (%) |
||||
|
Asymptomatic |
29 (21.6) |
105 (78.4) |
0.340 |
|
|
Mildly symptomatic |
40 (26.5) |
111 (73.5) |
||
Table 2: Risks of pneumonia by subgroups in 285 early-stage (asymptomatic or mildly symptomatic) COVID-19 patients in the control group without Andrographis paniculata treatment.
|
Potential risk variables |
Pneumonia, n (%) |
p-value |
|||
|
Yes |
No |
||||
|
AP treatment*, n (%) |
|||||
|
Yes |
1 (0.4) |
242 (99.6) |
<0.001 |
||
|
No |
69 (24.2) |
216 (75.8) |
|||
|
Age group (years), n (%) |
|||||
|
<50 |
53 (11.7) |
400 (88.3) |
0.009 |
||
|
51-60 |
17 (22.7) |
58 (77.3) |
|||
|
Sex |
|||||
|
Male |
26 (13.0) |
174 (87.0) |
0.892 |
||
|
Female |
44 (13.4) |
284 (86.6) |
|||
|
Body mass index categories, n (%) |
|||||
|
<25 kg/m2 (thin or normal) |
42 (11.8) |
315 (88.1) |
0.144 |
||
|
>25-35 kg/m2 (overweight or obese) |
28 (16.4) |
143 (83.6) |
|||
|
Presenting symptoms, n (%) |
|||||
|
-History of fever |
|||||
|
Yes |
13 (10.2) |
115 (89.8) |
0.235 |
||
|
No |
57 (14.2) |
343 (85.8) |
|||
|
-High body temperature (>37.5o C) |
|||||
|
Yes |
3 (18.8) |
13 (81.3) |
0.511 |
||
|
No |
67 (13.1) |
445 (86.9) |
|||
|
-Cough |
|||||
|
Yes |
23 (10.6) |
193 (89.4) |
0.141 |
||
|
No |
47 (15.1) |
265 (84.9) |
|||
|
-Nasal congestion or runny nose |
|||||
|
Yes |
12 (10.0) |
108 (90.0) |
0.231 |
||
|
No |
58 (14.2) |
350 (85.8) |
|||
|
-Myalgia |
|||||
|
Yes |
4 (7.5) |
49 (92.5) |
0.196 |
||
|
No |
66 (13.9) |
409 (86.1) |
|||
|
-Loss of smell |
|||||
|
Yes |
12 (9.2) |
118 (90.8) |
0.119 |
||
|
No |
58 (14.6) |
340 (85.4) |
|||
|
-Loss of taste |
|||||
|
Yes |
2 (4.8) |
40 (95.2) |
0.091 |
||
|
No |
68 (14.0) |
418 (86.0) |
|||
|
-Diarrhea |
|||||
|
Yes |
3 (13.0) |
20 (87.0) |
0.975 |
||
|
No |
67 (13.3) |
438 (86.7) |
|||
*AP = Andrographis paniculata
Table 3: The unadjusted risk of pneumonia for each potential risk variable in 528 early-stage (asymptomatic or mildly symptomatic) COVID-19 patients: A univariate analysis.
|
Point estimate |
(95% CI) |
|
|
Risks of pneumonia by AP treatment, % |
||
|
No |
24.3 |
(19.0, 29.7) |
|
Yes |
0.3 |
(0.0, 0.9) |
|
Risk difference, % |
24.0 |
(18.7, 29.4) |
|
Number needed to treat (NNT), n |
4 |
(3, 5) |
|
Number prevented when100 patients are treated, n |
24 |
(19, 29) |
NNT = 1 / risk difference; the number of patients that needed to treat for one patient not to have pneumonia
Table 4: Effect of Andrographis paniculata (AP) treatment on pneumonia development in adolescents and adults with early-stage (asymptomatic or mildly symptomatic) COVID-19, after adjustment for potential confounding effects.
4. Discussion
This study was performed in COVID-19 nonimmune adolescents and adults without comorbidities or pregnancy, during the second COVID-19 outbreak for three months in central region of Thailand. It demonstrated that the AP regimens, orally administered to early-stage COVID-19 patients within one week of the onset of symptoms, were safe and highly effective for the prevention of disease progression to moderate COVID-19 with pneumonia. These findings are in agreement with those of a previous study that reported a small, randomised, controlled trial involving adults with mild COVID-19[22]. Furthermore, a study performed in China demonstrated that a semi-synthetic injectable andrographolide preparation (Xiyanping) effectively improved the recovery of patients with mild-to-moderate COVID-19[23]. On the contrary, a recently published retrospective cohort study of real-world data from hospitalised patients, for a duration of 18 months (March 2020-August 2021), concluded that the use of the study AP crude extract was not associated with a decreased risk of pneumonia in mild COVID-19 patients[24].
The disadvantages of a retrospective cohort study are that the quality of the data which is generally inferior to that of a prospective study, selection bias may occur, and the presence of confounders may distort the association of the study variables. This study was performed right after a 3-month COVID-19 epidemic; the structured medical records of COVID-19 patients had been prepared for prospective recording. All eligible AP-treatment patients were included, and we randomly and blindly selected the control group to prevent selection bias. Additionally, the AP treatment and control groups were from the same population and matched by inclusion criteria and the existing potentially confounding effects were statistically adjusted.
Regarding generalizability, this study was performed in SARS-Co-V-2 unvaccinated population with early-stage COVID-19. We explored the characteristics of the control group and compared them with those of unvaccinated patients involved in other COVID-19 studies published in the literature. The finding that nearly half of the patients in our control group were asymptomatic at the time of admission is in agreement with the findings of another study performed in a similar setting in Chennai, India[25]. In our control group the occurrence of pneumonia was approximately 24%, which is comparable to the 20% occurrence rate of moderate and severe COVID-19 reported by a study of a large population in China[2]. During the study period, approximately 90% of the circulating strains in the included provinces belongs to the B.1.36.16 variant[26], and the COVID-19 vaccine was not yet available. The anti-SARS-CoV-2 effect of andrographolide may vary in different circulating strains.
There are a few possible explanations for the reduced risk of pneumonia during early-stage COVID-19 after AP treatment. First, andrographolide can inhibit intracellular SARS-CoV-2 replication[15]. Second, its anti-inflammatory effects are related to the reduced expression of interleukin-6 and tumour necrosis factor-alpha[16] which cause tissue damage. Third, it systemically distributes to various organs, including the lungs[27]. It was found that C-reactive protein, an inflammatory biomarker that is stimulated by interleukin-6, is related to the severity of COVID-19[28]. A previous small study in mild COVID-19 adults suggested its efficacy for preventing pneumonia, shortening of the viral shedding period, and suppressing C-reactive protein[22].
The AP treatment regimens in this study resulted in a few cases of mild gastrointestinal discomfort and non-specific mild symptoms. In addition, information regarding the acceptable safety of AP was determined by evaluating the long history of herbal medicine practices and recent reviews[6][29][7]. During animal studies, it was found that andrographolide has organ-protective effects on the liver, kidney, lung, and gastrointestinal tract. Toxicity is related to the concentration and administration time of andrographolide (long-term, high-dose, or rapid high-dose administration) [7].
In this COVID-19 nonimmune population, approximately one fourth of the patients in the control group developed pneumonia. The risk of pneumonia for asymptomatic patients was similar to mildly symptomatic patients. It is well-established that some asymptomatic patients are in pre-symptomatic phase and later develop symptoms[3]. The community-wide active case finding in the included provinces might be responsible for the very early detection of pre-symptomatic COVID-19 patients. All AP treatment patients in this study were symptomatic, further information on AP treatment for asymptomatic COVID-19 patients on the prevention of disease progression is required.
About 90% of the AP treatment patients in this study were treated with the AP extract regimen; therefore, we recommend the AP extract regimen (60 mg of andrographolide per dose, 3 times daily for 5 days). AP treatment should be started as soon as possible to effectively combat viral replication and inflammatory reactions that cause more severe diseases. The AP extract price is acceptably low, being only 100 to 300 Thai Baht (3-9 US dollar) per course of treatment. Furthermore, this oral medicine that occurs in nature is widely available. Thus this oral AP treatment is ideal for early-stage COVID-19 which is generally self-limited but could progress to more severe disease. Additional information is needed to explore its effects on children, patients older than 60 years, those with comorbidities, and pregnant women. Because AP is an herbal product, quality control and the standardisation of its preparations are important issues[30].
In conclusion, in addition to general supportive care, the oral AP treatment regimen administered to COVID-19 nonimmune adolescents and adult patients with early-stage COVID-19, and without comorbidities or pregnancy, are safe and very effective for preventing disease progression.
Declarations
Conflict of interest:
The authors declare no conflict of interest.
Funding:
This work was supported by Thai Traditional Medical Knowledge Fund, Department of Thai Traditional and Alternative Medicine, Ministry of Public Health; and Research Grant on Emerging Infectious Diseases, Faculty of Medicine, Siam University, Bangkok, Thailand [Grant number 003/2563].
Acknowledgement:
We thank the involved personnel at the study hospitals in Samut Sakorn, Nakhon Pathom, and Ratchaburi provinces, for their assistance in data collection.
CRediT authorship contribution statement:
AB: Conceptualisation, Project administration, Review, Funding acquisition, Editing & Resource. TS: Methodology, Review, Editing & Resource. TT: Methodology, Review, Editing & Resource. KW: Visualisation, Methodology, Review & Editing. PC: Formal analysis, Manuscript drafting, Review & Editing. CR: Formal analysis. SB: Formal analysis. SC: Formal analysis. SB: Formal analysis, Review & Editing. SL: Formal analysis, Visualisation, Review, Manuscript drafting, Editing & Funding acquisition. SS: Conceptualisation, Formal analysis, Visualisation, Manuscript drafting, Review & Editing.
Ethical approval:
The study was approved by the Department of Thai Traditional and Alternative Medicine, Ministry of Public Health, Thailand. Ethical approval was obtained from the Human Research Ethics Committee of Siam University (Reference Number: 2021/005). The data presented here were recorded during routine clinical practice. All data were de-identified prior to analysis and all the authors had all necessary administrative permissions to access the data.
Author Agreement
All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, has not received prior publication and is not under consideration for publication elsewhere.
Consent for publication:
Not applicable
References
- World Health Organization, 2022. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (Accessed 12 August 2022).
- Wu Z, McGoogan J M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA (2020); 323(13): 1239-1242.
- National Institutes of Health, USA. Clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. (Accessed 6 March 2023).
- Lamers M M, Haagmans B L, 2022. SARS-CoV-2 pathogenesis. Nat Rev Microbiol (2022); 20(5): 270-284.
- Kew Science, 2022. Plant of the World Online. Andrographis paniculata (Burm.f.) Nees. https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names: 45226-1. (Accessed 12 August 2022).
- Hossain S, Urbi Z, Karuniawati H, Mohiuddin R B, et al., Andrographis paniculata (Burm. f.) Wall. ex Nees: An updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life (Basel) (2021); 11(4): 348.
- Zeng B, Wei A, Zhou Q, Yuan M, et al., Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother Res (2022); 36: 336-364.
- Coon J T, Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta Med (2004); 70(4): 293-298.
- Poolsup N, Suthisisang C, Prathanturarug S, Asawamekin A,et al., Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther (2004); 29(1): 37-45.
- Shi T H, Huang Y L, Chen C C, Pi W C, et al., Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem Biophys Res Commun (2020); 533(3): 467-473.
- Sukardiman Ervina M, Fadhil Pratama M R, Poerwono H, Siswodihardjo S. The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Nees. J Adv Pharm Technol Res (2020); 11(4): 157-162.
- Enmozhi S K, Raja K, Sebastine I, Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J Biomol Struct Dyn (2021); 39(9): 3092-3098.
- Hiremath S, Kumar H D V, Nandan M, Mantesh M, et al., In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata, used in Ayurveda medicine in inhibiting SARS-CoV-2. 3 Biotech (2021); 11(2): 44.
- Murugan N A, Pandian C J, Jeyakanthan J. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J Biomol Struct Dyn. (2021); 39(12): 4415-4426.
- Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y, Thongsri P, et al., Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod (2021); 84(4): 1261-1270.
- Zhu N, Hou, J, Yang, N. Network pharmacology integrated with experimental validation revealed the anti-inflammatory effects of Andrographis paniculata. Sci Rep (2021); 11: 9752.
- Pholphana N, Panomvana D, Rangkadilok N, Suriyo T, et al., Andrographis paniculata: Dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers. J Ethnopharmacol (2016); 194: 513-521.
- Phumiamorn S, Sapsutthipas S, Pruksakorn P, Trisiriwanich S. In vitro study on antiviral activity of Andrographis paniculata against COVID-19. (2020); AAR_2563.pdf (in Thai). https://plandmsc.com.
- Yearsley C. Thailand approves Asian herb Andrographis to treat COVID-19. https://www.herbalgram.org/resources/herbalgram/issues/129/table-of-contents/hg129-wnews-thai-andro-cov19/. (Accessed 4 September 2021).
- Rajatanavin N, Tuangratananon T, Suphanchaimat R, Tangcharoensathien V. Responding to the COVID-19 second wave in Thailand by diversifying and adapting lessons from the first wave. BMJ Global Health (2021); 6: e006178.
- National Center for Biotechnology Information. National Library of Medicine, NIH. https://www.ncbi.nlm.nih.gov/search/all/?term=Andrographis%20paniculata. (Accessed 12 August 2022)
- Wanaratna K, Leethong P, Inchai N, Chueawiang W, et al., Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial. Arch Intern Med Res (2022); 5(3): 423-427.
- Zhang X Y, Lv L, Zhou Y L, Xie L D, et al., Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother Res (2021); 35(8): 4401-4410.
- Tanwettiyanont J, Piriyachananusorn N, Sangsoi L, Boonsong B, et al., Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised patients with mild coronavirus disease 2019: A retrospective cohort study. Front. Med (2022); 9: 947373.
- Krishnasamy N, Natarajan M, Ramachandran A, Vivian Thangaraj J W, et al., Clinical outcomes among asymptomatic or mildly symptomatic COVID-19 patients in an isolation facility in Chennai, India. Am J Trop Med Hyg (2021); 104(1): 85-90.
- COVID-19 Network Investigations (CONI) Alliance, 2021. Genomic epidemiology of novel coronavirus-Thailand-focused subsampling. https://nextstrain.org/community/fai-k/coni/Thailand?c=pango_lineage&l=scatter. (Accessed 12 August 2022).
- Songvut P, Suriyo T, Panomvana T, Rangkadilok N, et al., A comprehensive review on disposition kinetics and dosage of oral administration of Andrographis paniculata, an alternative herbal medicine, in co-treatment of coronavirus disease. Front Pharmacol (2022); 13: 952660.
- Mueller A A, Tamura T, Crowley C P, DeGrado J R, et al., Inflammatory Biomarker Trends Predict Respiratory Decline in COVID-19 Patients. Cell Rep Med. (2020); 1(8): 100144.
- Worakunphanich W, Thavorncharoensap M, Youngkong S, Thadanipon K, et al., Safety of Andrographis paniculata: A systematic review and meta-analysis. Pharmacoepidemiol Drug Saf (2021); 30(6): 727-739.
- Gagnier J J, Boon H, Rochon P, Moher D,et al., Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol (2006); 59(11): 1134-1149.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 