Patient Engagement is the Predominant Barrier to Completing Hepatitis C Virus Treatment in the Pan-Genotypic Direct-Acting Antiviral Era
Article Information
Clara Y. Tow, MD1,2, Meera Bhardwaj, MD3, Kazi Ullah, MD3, Jellyanna Peraza, MD4
Xianhong Xie, PhD2, Melissa Fazzari, PhD, MS2, Marouf Houssain, MD4,
Brett Fortune, MD, MS1,2
1Montefiore Medical Center, 111 E 210th Street, Bronx, NY 10467
2Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461
3Stony Brook Medicine, 101 Nicolls Road, Stony Brook, NY 11794
4Mount Sinai, 5 E 98th Street, New York, NY 10029
*Corresponding Author: Clara Y. Tow, MD, 1575 Blondell Ave, Ste 100, Bronx, NY 10461.
Received: 08 November 2023; Accepted: 16 November 2023; Published: 05 December 2023
Citation: Clara Y. Tow, MD, Meera Bhardwaj, MD, Kazi Ullah, MD, Jellyanna Peraza, MD, Xianhong Xie, PhD, Melissa Fazzari, PhD, MS, Marouf Houssain, MD, Brett Fortune, MD, MS. Patient Engagement is the Predominant Barrier to Completing Hepatitis C Virus Treatment in the Pan- Genotypic Direct-Acting Antiviral Era. Archives of Internal Medicine Research. 6 (2023): 129-137.
View / Download Pdf Share at FacebookAbstract
Background:
Data regarding real-world barriers patients encounter along the hepatitis C (HCV) care continuum is limited since the availability of pan-genotypic direct-acting antivirals (DAA). We sought to evaluate the HCV cascade of care during the pan-genotypic DAA era at an academic health system with multiple hospital and clinic sites in a large, diverse urban population.
Methods:
We conducted a retrospective cohort study of adult patients with chronic HCV at a single, academic health system between January 1, 2017 to September 30, 2019. The primary outcome evaluated was completion of DAA. Secondary outcomes included successful progression through each stage of the HCV care continuum from diagnosis to cure.
Results:
1215 patients were included. The average age was 61.5 years old and 62% were men. 84.5% had public insurance. All patients were referred to an HCV treatment provider. 550 patients (45.3%) met with an HCV treatment provider. 189 patients (15.6%) completed DAA. Active intravenous drug use, a mental health disorder, being referred by the emergency room or inpatient setting were associated with HCV treatment not being completed. Treatment by hepatology and infectious diseases and living closer to the treatment clinic was associated with treatment completion. Undergoing fibrosis staging, resistance testing, receiving medication education, and attending more clinic visits during the treatment course was also associated with treatment completion. Virologic response at 12 and 24 weeks was inconsistently obtained.
Conclusion:
In a predominantly under-represented minority, urban population with public insurance, significant barriers to HCV treatment with pan-genotypic DAAs continue to exist.
Keywords
Hepatitis C virus; viral hepatitis; Care cascade; Care continuum; Direct-acting antivirals
Article Details
Introduction
The release of pan-genotypic direct-acting antiviral (DAA) agents in 2016-2017 have allowed all patients with chronic hepatitis C virus (HCV) potential access to a cure.[1] While expensive, DAA oral availability, low side effect profile, cost-effectiveness, and reduction in HCV-related morbidity and mortality make them ideal.[2,3,4]
While scientific discovery and availability of DAA therapy were the first and biggest steps towards eradicating HCV, practical aspects regarding patient linkage to care and progression along the HCV care cascade remain the most important in achieving real-world success.[5] Studies have emerged over the years describing where patients fall off the HCV care cascade. These studies have been relatively small, lack granularity, and/or were performed outside the United States.[6,7,8] The most robust data available were obtained prior to the availability of pan-genotypic DAAs, had strict treatment criteria, or followed patients through the cascade based on where patients were initially diagnosed as opposed to how treatment was attempted.[9,10]
We sought to evaluate the HCV cascade of care at a single academic center with multiple hospital and clinic sites in a large, diverse urban population and determine the efficacy of various pathways based on the types of clinics and patient-provider relationships.
Methods and Materials
Study Design and Population
We conducted a retrospective cohort study of patients receiving care at a single, academic health system comprised of 3 major hospitals with affiliate primary and specialty clinics in Bronx, New York. Data was collected from January 1, 2017 to September 30, 2019. The starting date of data collection was selected as this was the time that pan-genotypic DAAs were readily available to patients in this geographic region. Patients included in the analysis were adults ³18 years old and had an HCV diagnosis defined by seropositivity and detectable RNA by HCV PCR, which is reflexive by protocol within our laboratory, during the study period. Patients already receiving treatment at the time of identification were excluded from the study. The study was approved by the Institutional Review Board of the study center. It was conducted in compliance with the Unite States Health Insurance Portability and Accountability Act.
Outcomes and Definitions
The primary outcome evaluated was completion of treatment, which was confirmed either by end of treatment (EOT) blood testing with HCV PCR or documentation within the electronic health records (EHR) of the patient completing treatment by a healthcare provider. Treatment completion was chosen over sustained virologic response at 12 (SVR12) or 24 weeks (SVR24) as cure rates for HCV are very high once treatment is completed, and compliance with SVR24 testing can be poor. Secondary outcomes included successful progression through each stage of the HCV care cascade from diagnosis to treatment completion and confirmation of a cure with SVR24 HCV PCR testing (Table 1).
|
Stage |
Definition |
Exclusion |
|
Diagnosis |
Patient had HCV antibody detection and detectable HCV RNA. |
Patient was already progressing through the HCV care continuum at the time of lab identification |
|
Referred |
Evidence of one of the following: |
No referral available or no documentation of discussion for patient to see a provider. |
|
Initial Assessment |
Patient presented for the first clinic visit with an HCV provider |
Patient never met an HCV provider |
|
Agreement to Pursue Treatment |
Documentation that HCV provider and patient agree to proceed with HCV treatment pending any necessary testing |
Documentation that the patient declines HCV treatment after meeting HCV provider |
|
Prior Authorization (PA) Submission |
Documentation from HCV provider that a PA was submitted to the insurance |
Documentation from HCV provider PA submission will not be pursued |
|
Medication Approval |
Documentation that DAA was approved by the insurance |
Documentation that DAA was not approved by the insurance |
|
Treatment Completed |
Confirmed administration of the entire prescribed treatment based on patient or family reporting |
Evidence of one of the following: reason |
|
End of Treatment (EOT) Response |
Undetectable HCV RNA at the completion of therapy |
Evidence of one of the following: HCV RNA |
|
Sustained Virologic Response at 12 weeks (SVR12) |
Undetectable HCV RNA at 12 weeks following completion of therapy |
Evidence of one of the following: HCV RNA |
|
Sustained Virologic Response at 24 weeks (SVR24) |
Undetectable HCV RNA at 24 weeks following completion of therapy |
Evidence of one of the following: HCV RNA |
Table 1: HCV Care Continuum: Definitions
Data Collection
All data were obtained from the EHR system utilized at our health system. Patient sociodemographic characteristics were collected at the time of diagnosis, including age, sex, shortest driving distance from home to the referred HCV clinic, preferred language, need for interpreter service, and primary insurance. Information regarding the characteristics of HCV infection were also collected: history of prior treatment, genotype, HCV RNA quantification at the time of diagnosis, fibrosis staging, and evidence of cirrhosis and decompensation. The DAA regimen and duration taken by patients was also recorded. Medical co-morbidities, specifically substance use disorder, tobacco use disorder, and mental health disorders, were noted.
Patients’ completion (or lack thereof) of every stage along the HCV care cascade was recorded. Specific dates were recorded (date of referral, date of the decision by the treatment provider to proceed with prior authorization application to the insurance company, and date of insurance approval). Other dates were omitted due to the inconsistent recording of events. Additional information, such as the specialty of the initial referring provider, whether the referring provider was within our health system or not, the medical specialty of the HCV treating provider, the type of treating provider (attending physician, housestaff, or advanced practice provider), testing performed prior to submitting prior authorization for treatment , number of office visits with the treating provider prior to submitting prior authorization, number of office visits during treatment, testing performed during treatment were collected. For patients who did not meet a treating provider after being diagnosed, notes were reviewed to see if a reason could be identified for not seeking treatment.
Statistical Analysis
For each step of the HCV care cascade, the proportion of patients completed was calculated. Categorical variables were expressed as number of patients and percentage and compared using Chi square test or Fisher’s exact test. Continuous variables were expressed as mean, median, standard deviation (SD), and interquartile range (IQR) and compared using two-sample t-test or Wilcoxon rank sum test. A p-value of < 0.05 was deemed statistically significant. All analyses were performed with SAS version 9.4.
Results
Patient Characteristics
During the study period, 1215 patients were identified to have HCV Ab positivity with detectable HCV PCR and were not already receiving DAA (Table 2). The average age was 61.5 years old and 62% were men. The majority of patients (84.5%) had public insurance and 30.6% had secondary insurance. 13.6% of patients had prior treatment exposure (9.1% with interferon and ribavirin, 4.5% with DAAs). About half of patients had unknown stage of fibrosis. For those with known fibrosis stage, the distribution of patients was: stage 0 = 9.7%, stage 1 = 8.6%, stage 2 = 8.7%, stage 3 = 6.7%, stage 4 =14.4%). Distribution was similar between those who completed DAA therapy and those who did not.
Active intravenous drug use was associated with HCV treatment not being completed (8.5% completed vs. 16.0% not completed, p=0.01). A mental health disorder noted in the history was also associated with treatment not being complete 33.3% completed vs. 36.6% not completed, p=0.0004), though the details of mental health disorders was not further defined (type of disorder, severity of disorder, whether disorders were under treatment or treatment-controlled).
|
Characteristics |
Overall (n=1215) |
Treatment completed (n=189) |
Treatment not completed (n=1026 |
p-value |
|
Age (years), mean (SD) |
61.5 (12.8) |
60.6 (11.7) |
61.6 (13) |
0.29 |
|
Sex, no. (%) |
0.26 |
|||
|
Male |
751 (62) |
124 (65.6) |
627 (61.3) |
|
|
Female |
461 (38) |
65 (34.4) |
396 (38.7) |
|
|
Primary insurance, no. (%) |
0.1 |
|||
|
Medicare |
474 (39.4) |
80 (42.3) |
394 (38.9) |
|
|
Managed Medicare |
70 (5.8) |
6 (3.2) |
64 (6.3) |
|
|
Medicaid |
466 (38.8) |
64 (33.9) |
402 (39.7) |
|
|
Managed Medicaid |
6 (0.5) |
1 (0.5) |
5 (0.5) |
|
|
Private |
167 (13.9) |
36 (19.1) |
131 (12.9) |
|
|
None |
19 (1.6) |
2 (1.1) |
17 (1.7) |
|
|
Preferred language |
0.02 |
|||
|
English |
1058 (87.2) |
153 (81.0) |
905 (88.3) |
|
|
Spanish |
139 (11.5) |
32 (16.9) |
107 (10.4) |
|
|
Other |
17 (1.4) |
4 (2.1) |
13 (1.3) |
|
|
Requires a language interpreter |
148 (12.2) |
32 (16.9) |
116 (11.3) |
0.03 |
|
Alcohol use disorder, no. (%) |
0.78 |
|||
|
None |
836 (68.8) |
132 (69.8) |
704 (68.6) |
|
|
Active |
199 (16.4) |
32 (16.9) |
167 (16.3) |
|
|
Former |
156 (12.8) |
23 (12.2) |
133 (13.0) |
|
|
Unknown |
24 (2.0) |
2 (1.1) |
22 (2.1) |
|
|
Intravenous drug use, no. (%) |
0.01 |
|||
|
None |
548 (45.1) |
85 (45.0) |
463 (45.1) |
|
|
Active |
180 (14.8) |
16 (8.5) |
164 (16.0) |
|
|
Former |
467 (38.4) |
87 (46.0) |
380 (37.0) |
|
|
Unknown |
20 (1.7) |
1 (0.5) |
19 (1.9) |
|
|
Tobacco use, no. (%) |
0.68 |
|||
|
Never |
283 (23.3) |
50 (26.5) |
233 (22.7) |
|
|
Active |
543 (44.7) |
79 (41.8) |
464 (45.2) |
|
|
Former |
366 (30.1) |
57 (30.2) |
309 (30.1) |
|
|
Unknown |
23 (1.9) |
3 (1.6) |
20 (2.0) |
|
|
Mental health disorder, no. (%) |
438 (36.1) |
66 (33.3) |
375 (36.6) |
0.0004 |
|
Referring provider, no. (%) |
<0.0001† |
|||
|
Primary care |
369 (30.4) |
113 (59.8) |
256 (25.0) |
|
|
Infectious disease |
47 (3.9) |
17 (9.0) |
30 (2.9) |
|
|
Gastroenterology |
27 (2.2) |
8 (4.2) |
19 (1.9) |
|
|
Emergency room/hospital |
131 (10.8) |
14 (7.4) |
117 (11.4) |
|
|
Self-referral |
8 (0.7) |
5 (2.7) |
3 (0.3) |
|
|
Hepatology |
60 (4.9) |
20 (10.6) |
40 (3.9) |
|
|
Other |
149 (12.3) |
10 (5.3) |
139 (13.6) |
|
|
Unknown |
423 (34.8) |
2 (11) |
421 (41.1) |
SD, standard deviation; no., number; †p<0.00001 when the “unknown” category is excluded
Table 2: Baseline characteristics of patients with chronic HCV
HCV Care Cascade
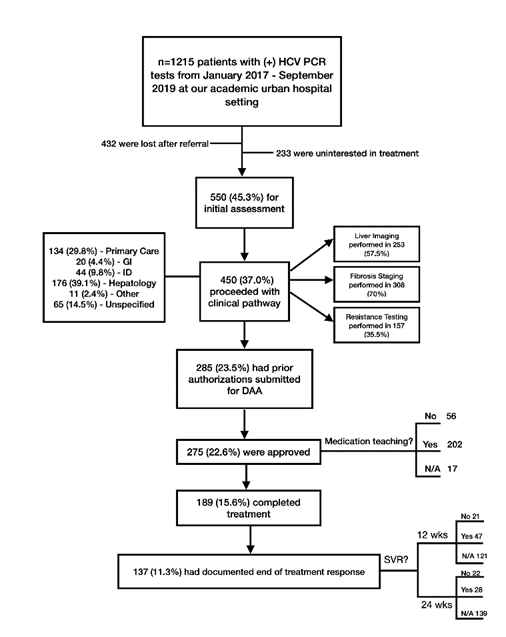
All 1215 patients were referred to an HCV treatment provider. Their progression along the HCV care cascade is summarized in Figure 1. The plurality of patients was identified and referred by primary care providers (30.4%), followed by emergency room and inpatient hospital settings (10.8%), hepatology (4.9%), infectious disease (3.9%), gastroenterology (2.2%), and then self-referred (0.7%). 12.3% of patients were referred by other providers, of which the majority were in solid organ or bone marrow transplant or oncology. For the largest group of patients (34.8%), it was unclear who diagnosed and referred the patients for treatment. Being referred by the emergency room, inpatient hospital setting, an “other” provider not within the above listed specialties, or “unknown” provider was associated with treatment not being completed (p<0.0001).
Of the 1215 patients identified, 550 patients (45.3%) met with an HCV treatment provider. HCV treatment providers were available at different clinics: primary care, infectious disease, hepatology, gastroenterology, other (often transplant clinics), and unspecified. Ultimately only 189 (15.6%) of patients completed treatment. Patient progression through the HCV care cascade based on the type of treatment provider was similar amongst the various specialties.
Being treated by hepatology and infectious diseases specialty clinics and living closer to the treatment clinic (3.2 vs. 3.7 miles, p=0.01) was associated with treatment completion (Table 3). Patients who completed treatment more often underwent fibrosis staging (86.2% of treatment completed vs. 57.9% of treatment not completed, p<0.0001), resistance testing (42.3% of treatment completed vs. 30.3% of treatment not completed, p=0.01), and trended towards more non-fibrosis liver imaging (62.7% vs. 53.7%, p=0.06). Furthermore, patients were more successful when provided medication education prior to the taking DAA (80.9% vs. 62.5%, p=0.001) and attending more clinic visits during the treatment course (2 visits vs. 1 visit, p<0.0001).
|
Characteristics |
Overall (n=450) |
Treatment completed (n=189) |
Treatment not completed (n=261) |
p-value |
|
Clinic setting, no. (%) |
<0.0001‡ |
|||
|
Primary care |
134 (29.8) |
60 (31.8) |
74 (28.4) |
|
|
Gastroenterology |
20 (4.4) |
11 (5.8) |
9 (3.5) |
|
|
Infectious disease |
44 (9.8) |
27 (14.3) |
17 (6.5) |
|
|
Hepatology |
176 (39.1) |
83 (43.9) |
93 (35.6) |
|
|
Other |
11 (2.4) |
7 (3.7) |
4 (1.5) |
|
|
Unknown |
65 (14.5) |
1 (0.5) |
64 (24.5) |
|
|
Distance from home to treatment provider (miles), median (IQR) |
3.5 (2.5.4) |
3.2 (1.5.1) |
3.7 (2.2-5.5) |
0.01 |
|
Liver imaging performed prior to treatment, no. (%) |
253 (57.5) |
116 (62.7) |
137 (53.7) |
0.06 |
|
Fibrosis staging performed prior to treatment, no. (%) |
307 (70.0) |
162 (86.2) |
146 (57.9) |
<0.0001 |
|
Resistance testing performed prior to treatment, no. (%) |
157 (35.5) |
80 (42.3) |
77 (30.3) |
0.01 |
|
No. of visits prior to PA submission, median (IQR) |
1 (1-2) |
1 (1-2) |
1 (1-2) |
0.48 |
|
Time from initial visit to PA submission (days), median (IQR) |
74 (22-227) |
93 (17-204) |
72 (22-227) |
0.87 |
|
Reasons for delay in PA submission, no. (%) |
0.01 |
|||
|
Patient compliance |
18 (5.1) |
11 (5.9) |
7 (4.2) |
|
|
Testing incomplete |
92 (26.2) |
59 (31.7) |
33 (20.0) |
|
|
Patient co-morbidities |
27 (7.7) |
8 (4.3) |
19 (11.5) |
|
|
Patient hospitalization |
6 (1.7) |
2 (1.1) |
4 (2.4) |
|
|
Other |
39 (11.1) |
14 (7.5) |
25 (15.2) |
|
|
Multiple |
3 (0.9) |
2 (1.1) |
1 (0.6) |
|
|
No delay |
166 (47.3) |
90 (48.4) |
76 (46.1) |
|
|
Time from PA submission to medication approval (days), median (IQR) |
27 (156.5) |
27 (12-56) |
27 (12-66) |
0.92 |
|
Approved regimen, no. (%) |
0.97 |
|||
|
Elbasvir/grazoprevir |
16 (6.0) |
11 (5.9) |
7 (6.0) |
|
|
Glecaprevir/pibrentasvir |
164 (54.3) |
99 (53.2) |
65 (56.0) |
|
|
Ledipasvir/sofosbuvir |
55 (18.2) |
34 (18.3) |
21 (18.1) |
|
|
Daclatasvir/sofosbuvir |
2 (0.7) |
2 (1.1) |
0 (0.0) |
|
|
Paritaprevir/ritovir/ombitasvir/dasabuvir |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Daclatasvir/sofosbuvir |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Sofosbuvir/velpatasvir |
62 (20.5) |
39 (21.0) |
23 (19.8) |
|
|
Sofosbuvir/simepravir |
1 (0.3) |
1 (0.5) |
0 (0.0) |
|
|
Medication teaching performed, no. (%) |
271 (74.3) |
152 (80.9) |
65 (62.5) |
0.001 |
|
No. of visits during treatment, median (IQR) |
2 (1-3) |
2 (1-3) |
1 (1-2) |
<0.0001 |
IQR, interquartile range; PA, prior authorization, ‡p<0.00001 when the unknown category is excluded.
Table 3: Clinical pathway of HCV patients agreeing to proceed with HCV treatment
Virologic response was poorly tracked (Table 4). The vast majority of patients had EOT testing performed (n=137, 72.5% of the treated cohort) and all had undetectable viral loads. Only 68 patients had SVR12 and 60 patients had SVR24 testing (30.6% and 11.6% of the treated cohort, respectively). Of the patients who completed treatment and were tested, 22 patients did not achieve SVR 24 (11.5% of the treated cohort). There was no statistical difference in virologic response tracking or achieving SVR24 amongst the different clinics.
|
Virologic Response |
Treatment completed (n=189) |
|
End of treatment response, no. (%) |
|
|
Yes |
137 (72.5) |
|
No |
0 (0.0) |
|
Not checked |
52 (27.5) |
|
Sustained virologic response at 12 weeks, no. (%) |
|
|
Yes |
47 (24.9) |
|
No |
21 (11.1) |
|
Not checked |
121 (64.0) |
|
Sustained virologic response at 24 weeks, no. (%) |
|
|
Yes |
28 (14.8) |
|
No |
22 (11.6) |
|
Not checked |
139 (73.5) |
Table 4: Virologic response among patients who completed HCV treatment
Patients Who Did Not Seek HCV Treatment
432 (35.5%) did not follow-up with any treatment provider and 233 (19.2%) had told their referring provider they were uninterested in treatment. Of the patients who met with a HCV treatment provider, an additional 100 patients (8.2%) opted to not proceed with treatment. More granular data about the reasons for not seeking treatment were difficult to obtain.
Patients Who Did Not Have a Prior Authorization Submitted
Additional analysis was performed to identify any sociodemographic and clinical factors that differentiated patients who successfully proceeded along the care cascade to have a prior authorization submitted versus those who did not (Supplemental Table 1). Patients with active intravenous drug use were less likely to have a prior authorization submitted (16.8% vs. 8.4%). On the other hand, patients with former intravenous drug use were more likely to proceed along the care cascade and have a prior authorization submitted (47.4% vs. 35.7%). Patients with prior HCV treatment more often completed testing and clinic visits in order to have a prior authorization submitted (20.8% vs. 11.8%, p=0.0001). The type of provider who initially identified and referred the patient for treatment also had an impact on whether patients reached the stage of a prior authorization submitted.
Discussion
The present real-world study demonstrates that in a predominantly under-represented minority, urban population with public insurance, significant barriers to HCV treatment with pan-genotypic DAAs continue to exist. While all patients with chronic HCV were identified by a healthcare provider and subsequently referred to an HCV treatment provider, only 15.6% successfully completed treatment. This is the largest study to date evaluating patients progressing through the HCV care cascade and the only to evaluate treatment since the advent of pan-genotypic DAAs.
All patients with HCV antibody detection were confirmed with HCV RNA polymerase chain reaction (PCR). 100% of patients found to have chronic HCV infection were electronically referred to an HCV treatment provider. Prior studies have reported lower levels of confirmatory testing and subsequent referrals. A recent study published in 2018 at a single US academic center cited only 79% of patients receiving confirmatory RNA testing. Other studies conducted at federally qualified health centers in Philadelphia had confirmatory testing rates between 84-95%.[9] Subsequent referrals rates ranged between 75-90%. Younger patients and those with active substance use disorders were less often referred.
At our health system, all age-appropriate patients are flagged in the inpatient and outpatient HER for HCV screening. Blood samples that test positive for HCV antibody reflex test for HCV RNA PCR. Abnormal results are subsequently flagged in the provider’s inbox. These automated systems bypass several human steps needed to correctly identify patients with active infection and easily allow providers to submit electronic referrals for all patients regardless of sociodemographic factors. These interventions have allowed our system to have 100% care linkage to an HCV treatment provider and have proven highly beneficial in increasing HCV diagnosis and referrals other studies in the United States and abroad.[11,12]
The most common barriers to completing HCV treatment related to active patient engagement along the care çascade. The largest drop-off in patient engagement was getting patients to meet with an HCV treatment provider. While all patients were referred, 54.7% never came to an initial appointment with an HCV treatment provider. Patients referred from the inpatient setting were less likely to pursue and complete treatment. Granular details regarding this particular issue are lacking, though could be due to lack of patient education, poor patient-physician relationships, difficulty coordinating care between inpatient and outpatient settings, and/or to low priority of treating a chronic condition that is often asymptomatic.
During the interferon era, poor attendance rates were observed with only 43-76% of patients attending their first appointment with an HCV treatment provider.[13,14,15] More recent studies that included patients during the early DAA era have reported similarly wide ranges of initial attendance rates between 33-70%.[16,17,18] Most studies with higher attendance rates studied fewer clinics within a narrow geographic region, included clinics of one or few medical specialties, and/or only included outpatient clinics. The largest study evaluating 885 chronically infected patients associated with federally qualified health centers in Philadelphia between 2012-2016 had an initial attendance rate of 69.4%.[9]
In this study, reasons patients declined to see an HCV treatment provider were poorly documented. More than one-third of these patients cited disinterest in pursuing treatment despite counseling received by the referring provider. Reasons, when documented, included concerns about the treatments themselves, the duration and commitment to treatment, the patients’ view of their other co-morbidities, and/or whether treatment would have an overall impact on their quality or quantity of life. Of the patients who presented for initial assessment with an HCV treatment provider, 18.1% (8.2% of all HCV patients) decided against further evaluation and testing for HCV treatment. For this group of patients, the decision, when documented, was often made by the patient and physician together and took into consideration issues of quality and quantity of life. Qualitative studies have tried to look at this patient perspective in further detail; however, their extremely small sample sizes and unique patient populations make it challenging to extrapolate for real-world application.[19,20,21] Larger, more inclusive studies are needed to better inform how to better engage patients to seek HCV treatment.
With the availability of pan-genotypic DAAs, genotype and resistance testing and fibrosis staging are often not required in order to prescribe therapy. At our health system, individual practitioners (regardless of their clinic affiliation) required different degrees of testing prior to submitted a prior authorization for DAA therapy. Testing requirements may be seen as a barrier to care for many patients. Yet in our study, patients who underwent fibrosis testing (with labs or imaging), resistance testing, and imaging were associated with treatment completion compared to those who did not. Furthermore, more clinic visits and medication teaching prior to initiating DAA therapy was also associated with treatment completion. These findings suggest that having patients engage with the health center and medical team may improve patient engagement and treatment completion.
Once DAAs were approved by insurance, there remained a large drop-off in patient follow-up to confirm treatment was completed (31% of patients were not confirmed to have completed treatment) and assess SVR. Similar loss of patients after treatment was prescribed and prior authorization approved has been seen in other studies, particularly in urban clinics.[9,10] It may be presumed that a proportion of these patients did ultimately complete treatment. Prior studies have not reached out to such patients to determine the ultimately treatment completion rate. However, the Telepass project reported that about 19% of recalled patients who were documented as having chronic HCV and not treated had actually successfully completed treatment.[22]
Many of the limitations to this study relate to its retrospective nature. Data as to why patient engagement was poor leading to failed progression along the care cascade are lacking. The HCV screening and treatment algorithms are highly variable among the different hospitals and clinics within the health system as well as among the individual referring and treatment providers. While our health system has a significant catchment area, the majority of the population served are urban, under-represented minority patients with public insurance; thus results may not be applicable broadly.
Despite scientific advancements that have allowed us to cure almost all patients with chronic HCV, the dissemination and completion of DAA treatment remain significant challenges. Pan-genotypics have not changed barriers to care linkage and patient progression along the HCV care cascade. Past studies have not informed current health systems how to successfully overcome identified breaks in the care cascade as our study shows that the vast majority of patients remain uncured. Further research and health system efforts must focus on how to increase patient engagement. Areas of interest should include improving care linkage between inpatient and outpatient settings, increasing patient education and awareness of HCV treatments, increasing interactions between medical providers and patients during the evaluation and treatment process.
Acknowledgements
None
Conflicts of Interest
None
References
- AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Retrieved July, from http://wwww.hcvguidelines.org. (2022).
- Jung J, Feldman R, Kalidindi Y, et al. Association of direct-acting antiviral therapy for hepatitis C with after-treatment costs among Medicare beneficiaries. JAMA Netw Open 3 (2020): e208081.
- Kalidindi Y, Jung J, Feldman R, Riley 3rd T. Association of direct-acting antiviral treatment with mortality among medicare beneficiaries with hepatitis C. JAMA Netw Open 3 (2020): e2011055.
- Park H, Wang W, Henry L, et al. Impact of all-oral direct-acting antivirals on clinical and economic outcomes in patients with chronic hepatitis C in the United States. Hepatology 69 (2019):1032-1045.
- Lombardi A, Mondelli MU, ESCMID Study Group for Viral Hepatitis (ESGVH). Hepatitis C: is eradication possible? Liver Int. 2019;39 (2019): 416-426.
- Petroff D, Bätz O, Jedrysiak K, et al. From screening to therapy: anti-HCV screening anid linkage to care in a network of general practitioners and a private gastroenterology practice. Pathogens 10 (2021): 1570.
- Reyes-Urueña J, Celly A, Moreno S, et al. Hepatitis C virus: testing rate and attrition at linkage to specialized care, Catalonia, Spain 2011-2016. J Viral Hepat 28 (2021): 288-299.
- Malespin M, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with diret acting antivirals in an urban clinic. Ann Hepatol 18 (2019): 304-309.
- Coyle C, Moorman AC, Bartholomew T, et al. The hepatitis C virus care continuum: linkage to hepatitis C virus care and treatment among patients at an urban health network, Philadelphia, PA. Hepatology 70 (2019): 476-486.
- Calner P, Sperring H, Ruiz-Mercado G, et al. HCV screening, linkage to care, and treatment patterns at different sites across one academic medical center. PLoS One 14 (2019): e0218388.
- Al-Hihi E, Shankweiler C, Stricklen D, et al. Electronic medical record alert improves HCV testing for baby boomers in primary care setting: adults born during 1945-1965. BMJ Open Qual 6 (2017): e000084.
- Goel A, Sanchez J, Paulino L, et al. A systematic model improves hepatitis C virus birth cohort screening in hospital-based primary care. J Viral Hepat 24 (2017): 477-485.
- Groom H, Dieperink E, Nelson DB, et al. Outcomes of a hepatitis C screening program at a large urban VA medical center. J Clin Gastroenterol 42 (2008): 97-106.
- Putka B, Mullen K, Birdi S, et al. The disposition of hepatitis C antibody-positive patients in an urban hospital. J Viral Hepat 16 (2009): 814-821.
- Butt AA, Wagener M, Shakil AO, et al. Reasons for non-treatment of hepatitis C in veteranas in care. J Viral Hepat 12 (2005): 81-85.
- Blanding DP, Moran WP, Bian J, et al. Linkage to specialty care in the hepatitis C care cascade. J Investig Med 69 (2021): 324-332.
- Zuckerman A, Douglas A, Nwosu S, et al. Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One 13 (2018): e0199174.
- Saab S, Challita YP, Najarian LM, et al. Hepatitis C screening: barriers to linkage to care. J Clin Transl Hepatol 7 (2019): 226-231.
- Sherbuk JE, Tabackman A, McManus KA, et al. A qualitative study of perceived barriers to hepatitis C care among people who did not attend appointments in the non-urban US South. Harm Reduct. J 17 (2020): 64.
- Wang R, Cui N, Long M, et al. Barriers to uptake of hepatitis C virus (HCV) health intervention among men who have sex with men in Southwest China: a qualitative study. Health Soc Care Community 29 (2021): 445-452.
- Assoumou SA, Sian CR, Gebel CM, et al. Patients at a drug detoxificationi center share perspectives on how to increase hepatitis C treatment uptake: a qualitative study. Drug Alcohol Depend 220 (2021): 108526.
- Ponziani FR, Santopaolo F, Siciliano M, et al. Missed linkage to care for patients who screened positive for hepaitis C in a tertiary care centre: results of the telepass project. J Viral Hepat 28 (2021): 651-656.
Supplementary data
Supplemental Table 1:
Characteristics of patients who did not meet HCV treatment provider or declined to proceed with HCV treatment compared to patients who decided to pursue HCV treatment.
|
Characteristics |
Never saw provider, declined treatment, or incomplete testing (no PA) (n=930) |
Completed testing to obtain PA (n=285) |
p-value |
|
Age (years), mean (SD) |
61.5 (13.1) |
61.4 (11.9) |
0.87 |
|
Primary insurance, no. (%) Medicare Managed Medicare Medicaid Managed Medicaid Private None |
365 (39.8) 46 (5.0) 366 (39.9) 5 (0.6) 118 (12.9) 17 (1.9) |
109 (38.3) 24 (8.4) 100 (35.1) 1 (0.4) 49 (17.2) 2 (0.7) |
0.06 |
|
Alcohol use disorder, no. (%) None Active Former Unknown |
645 (69.4) 151 (16.2) 113 (12.2) 21 (2.3) |
191 (67.0) 48 (16.8) 43 (15.1) 3 (1.1) |
0.35 |
|
Intravenous drug use, no. (%) None Active Former Unknown |
423 (45.5) 156 (16.8) 322 (35.7) 19 (2.0) |
125 (43.9) 24 (8.4) 135 (47.4) 1 (0.4) |
<0.0001 |
|
Tobacco use, no. (%) Never Active Former Unknown |
210 (22.6) 425 (45.7) 274 (29.5) 21 (2.3) |
73 (25.6) 118 (41.4) 92 (32.3) 2 (0.7) |
0.16 |
|
Mental health disorder, no. (%) |
342 (36.8) |
96 (33.7) |
0.87 |
|
History of prior HCV treatment, no. (%) |
109 (11.8) |
59 (20.8) |
0.0001 |
|
Liver fibrosis stage 0/4 1/4 2/4 3/4 4/4 Unknown |
63 (6.7) 49 (5.2) 44 (4.7) 49 (5.2) 111 (11.9) 614 (66.0) |
54 (19.0) 54 (19.0) 60 (21.1) 31 (10.9) 62 (21.8) 24 (8.4) |
<0.0001§ |
|
Initial referring provider, no. (%) Primary care Infectious disease Gastroenterology Hospitalization Self-referral Hepatology Other Unknown |
192 (20.8) 21 (2.3) 12 (1.3) 112 (12.0) 1 (0.1) 39 (4.2) 134 (14.4) 418 (45.0) |
176 (62.0) 26 (9.2) 15 (1.5) 19 (6.7) 7 (2.5) 21 (7.4) 15 (5.3) 6 (1.8) |
<0.0001¶ |
§ p=0.003 when the “unknown” category is excluded.
¶p-value remains the same when the “unknown” category is excluded.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 