Species Composition of Culicidae (Diptera) in Bromeliads in rural and urban areas of Londrina, Brazil
Article Information
Bianca Piraccini Silva1, Adriano Nobre Arcos2, Francisco Augusto da Silva Ferreira3,
Cristiano Medri4, João Antonio Cyrino Zequi*, 5
1Laboratory of Medical Entomology, Department of Animal and Plant Biology, Universidade Estadual de Londrina (UEL), Post-graduate Program in Biological Sciences, Londrina, PR, Brazil.
2Hydrology Laboratory (CODAM/LBA), Malaria and Dengue Laboratory (COSAS), Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, AM, Brazil
3Graduate Program in Biological Sciences (Entomology), Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, AM, Brazil
4Laboratory of Plant Morphology and Anatomy, Universidade Estadual de Londrina (UEL), Londrina, PR, Brazil
5Laboratory of Medical Entomology, Department of Animal and Plant Biology, Universidade Estadual de Londrina (UEL), Post-graduate Program in Biological Sciences, Londrina, PR, Brazil
*Corresponding author: João Antonio Cyrino Zequi, Laboratory of Medical Entomology, Department of Animal and Plant Biology, State University of Londrina (UEL), Post-graduate Program in Biological Sciences, Londrina, PR, Brazil.
Received: 26 October 2023; Accepted: 05 March 2024; Published: 12 April 2024
Citation: Silva, B.P.; Arcos, A.N.; Ferreira, F.A.S.; Medri, C., Zequi, J.A.C. Species Composition of Culicidae (Diptera) in Bromeliads in rural and urban areas of Londrina, Brazil. Archives of Microbiology and Immunology. 8 (2024): 118-124.
View / Download Pdf Share at FacebookAbstract
Bromeliaceae has 82 genera and 3,719 species. Culicidae use phytotelma as a breeding ground. Research on the importance of bromeliads and reproduction of mosquitoes are controversial. Some studies indicate that these plants are potential breeding grounds for Aedes, while others suggest that they are not preferential foci for synanthropic mosquitoes, as is the case for Aedes aegypti. This work aimed at verifying the presence of Aedes and other Culicidae in native bromeliads in natural habitat and under ornamental cultivation in an urban environment, comparing the incidence of larvae in these possible breeding sites and contrasting with the luminosity and accumulated water volumes. Differences in species composition were observed between rural and urban environments. In the rural environment, four species of Culicidae were collected, the most abundant being Wyeomyia galvaoi - 19 specimens, followed by Culex (Microculex) imitator - five specimens, while Ae. aegypti and Toxorhynchites sp 1, were the least abundant, with two individuals each. In the urban environment, five species were found, with greater abundance of Ae. aegypti (461 individuals), followed by Aedes albopictus (45), Toxorhynchites sp. 01 (3), Wyeomyia sp1 (02), and Wyeomyia galvaoi (01). These phytotelma showed a great diversity of other organisms, such as mites, nematodes, protists, rotifers, ostracods, and insect larvae of the Chironomidae, Syrphidae, and Tachinidae families. The study that mosquito larvae in native bromeliads are generally rare, and their presence can be affected by factors like light and water volume. The significance of bromeliads in mosquito reproduction depends on various factors.
Keywords
Bromeliaceae, Neotropical region Tank bromeliads, Phytotelmata, Mosquito breeding site
Article Details
1. Background
The Bromeliaceae family comprises 82 genera and 3,719 species, widely dispersed in the Neotropical region, extending from the southern United States to central Argentina and Chile. These plants are present in environments with different characteristics, from extremely dry and sunny to humid and shady, and at different altitudes and temperatures [1,2]. Only one species, Pitcairnia feliciana (A.Chev.) Harms & Mildbr., is found on the African continent. These plants are present in all Brazilian biomes, especially in the Atlantic Forest, where they represent one of the most relevant taxonomic groups, due to the high degree of endemism. The Bromeliaceae family has important ecological value, mainly due to its interactions with fauna, constituting a micro-habitat for several species of vertebrates and invertebrates [3,4]. In this context, the tank bromeliads stand out, presenting ecological interactions that occur due to the morphology of the species, which are endowed with rosette phyllotaxis and arranged leaf sheaths, forming permanent water accumulator compartments called phytotelmata, that are rich in organic debris and living organisms [1]. The water accumulated in the plants is used as a water trough, foraging site, for shelter, and refuge from predators, as well as for breeding and nesting sites [5]. It is also noteworthy that these tanks are places of oviposition and larval development for several orders of insects, including mosquitoes of the Culicidae family.
Culicidae use phytotelma as a breeding ground, in this way the accumulated water is utilized as a place for the development of their immature forms. Some genera even have a high dependence on these tanks, such as some species of Wyeomyia [6]. The importance of bromeliads as water reservoirs that can favor the reproduction of mosquito vectors of pathogens is controversial. Research has produced contradictory results on the findings of immature forms of Aedes aegypti Linnaeus and Aedes albopictus Skuse in their phytotelma. Some studies point out that these plants are potential breeding grounds for Aedes [7-13]. On the other hand, some findings indicate that bromeliads are not the preferential foci of synanthropic mosquitoes, as is the case with Ae. aegypti [6,14-18]. The authors emphasize that bromeliads form a complex scenario, in which their importance in mosquito reproduction can vary according to the location, habitat, climate, biotic and abiotic characteristics of the phytotelma, and species of vector and bromeliad, in addition to human behavior. Given the above, the current work sought to verify the presence of Aedes and other Culicidae in native bromeliads occurring in the natural habitat and under ornamental cultivation in an urban environment; comparing the incidence of larvae in these possible breeding sites and contrasting with the luminosity and accumulated volumes of water; seeking to answer if, and under what conditions, these plants can be breeding grounds for Aedes larvae and other Culicidae.
2. Material and Methods
Study area
The study was conducted in two locations in the city of Londrina, Paraná state, southern Brazil, a region with a Cfa (subtropical) climate, according to the Köppen classification, with an average temperature in the coldest month of below 18°C and average in the hottest month of above 22°C [19].
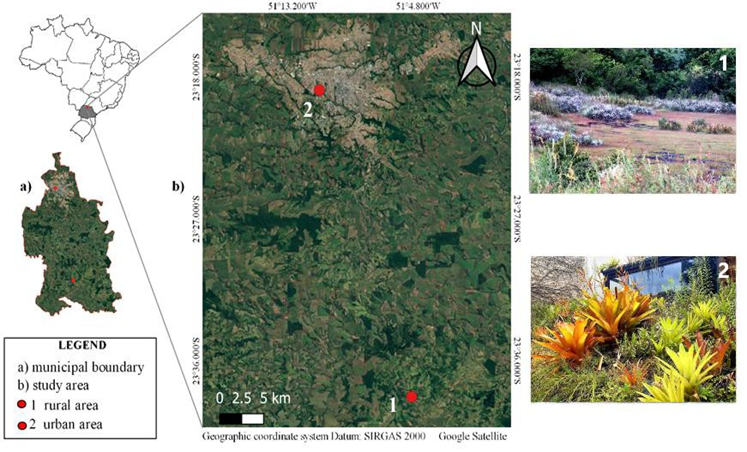
The first location (23°38'17.44"S 51° 5' 12.48"W; with 711 meters) (Google Earth LCC) is in a rural area with basaltic outcrops covered by rupicolous and endemic vegetation, composed, in addition to other species and families of the bromeliads Dyckia walteriana Leme, Dyckia leptostachya Baker, and Bromelia balansae Mez., as well as the tank bromeliad Aechmea distichantha Lem. The second location is in a residence in an urban area (23° 18 '49.13 ``S, 51° 11' 24.51 ``W; with 553 meters) (Google Earth LCC); containing a sunny part of the garden consisting of a green roof and a ground floor area, with cultivation sites for the tank bromeliads Alcantarea imperialis (Carriere) Harms, Aechmea blanchetiana (Baker) LB Sm. var. rubra, A. blanchetiana var. limão, A. distichantha, Aechmea pineliana (Brong. Planch.) Baker., and Neoregelia cruenta (R.Graham) LB Sm.; and a shady part of the garden composed of a sloping area and a flat area, places where the following tank bromeliads are cultivated A. blanchetiana var. rubra, Aechmea nudicaulis (L.), Neoregelia sp. (híbrida), and Vriesea guttata Linden & André. Of all the species cultivated in the residence in the urban area, only A. distichantha is native to the region of Londrina, PR (Figure 1).
Material collection
Sampling was carried out in two locations between March and April 2017, weekly, for five consecutive weeks. Thermo-hygrometers were installed at both locations to measure the temperature (°C) and relative humidity (%). In addition, the rainfall data measured by the Londrina meteorological station during the experimental period were obtained from the Paraná Meteorological System (SIMEPAR). As a way of monitoring the presence of Aedes, traps to capture eggs (ovitraps) were installed in each location. At point 1, five individuals of A. distichantha were monitored, and at point 2, five individuals of each species/environment (sun or shade) mentioned above, except for A. imperialis, for which three individuals were sampled as this was the total number available. During this period, water from the bromeliads was extracted with the aid of a silicone tube attached to a syringe. The life forms found were collected with a sieve (1mm mesh) and the volume of water was measured using a beaker. The water was later returned to the tank bromeliads. The collected life forms were taken for identification and counting with the aid of optical microscopy, together with samples of the water accumulated in each bromeliad, to the Laboratory of General and Medical Entomology of the State University of Londrina. Identification of the taxonomic groups collected was carried out, as well as the number of individuals collected in each group, with emphasis on the larval forms of Ae. aegypti and Ae. albopictus, in addition to possible other Culicidae. The water accumulated in the bromeliads was analyzed by measuring pH, conductivity, and temperature.
Data analysis
The data obtained for A. distichantha were compared between the two collection points. At point 2, data were compared between sunny and shaded environments, between species, and between plants that accumulate more than one liter of water with those that accumulate less than one liter. For both collection points, the data obtained were tested in relation to the correlation between the physical-chemical water and meteorological variables during the experimental period. Multiple linear regression was used to verify the relationship between the dependent variable (abundance of mosquitoes) and the independent variables (volume, pH, conductivity). The relationships between the larval composition of mosquitoes and environmental variables were evaluated by means of exploratory canonical redundancy analysis (CRA) [20] using the Monte Carlo test, with 9999 unrestricted permutations to determine the significance level of the variables [21]. The CRA, with only the selected variables, served as a model for the analysis of variance partition [22], simulated with a Venn diagram. This analysis indicates how much of the variation in species composition is explained by the different components and by the interaction between the components [23] within the model. All analyses are available in the Vegan package and performed in R [24,25].
3. Results
Differences in species composition were observed between rural and urban environments. In the rural environment, four species of Culicidae were collected, the most abundant being Wyeomyia galvaoi [26] with 19 specimens, followed by Culex (Microculex) imitator Theobald, 1903 with five specimens, while Ae. aegypti (Linnaeus, 1762) and Toxorhynchites sp 1, were the least abundant, with two individuals each. On the other hand, in the urban environment, five species were found, with greater abundance of Ae. aegypti (461 individuals), followed by Ae. albopictus (Skuse, 1894) (45), Toxorhynchites sp. 01 (3), Wyeomyia sp1 (02), and Wy. galvaoi (01) (Figure 1). In addition, these phytotelma showed great diversity of other organisms, such as mites, nematodes, protists, rotifers, ostracods, and insect larvae of the Chironomidae, Syrphidae, and Tachinidae families.
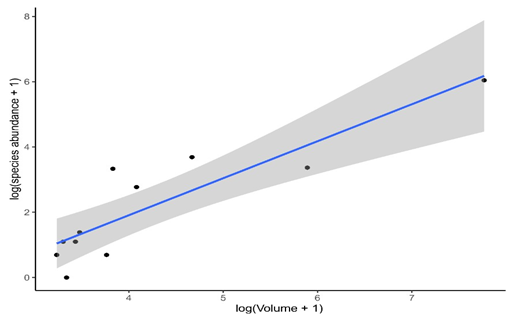
Multiple linear regression analysis revealed that the abundance of Culicidae in bromeliads was significantly correlated with the volume of water in the phytotelma (t- value= 5.083; p-value= 0.00143) (Figure 2). However, limnological characteristics such as conductivity and pH were not correlated with the abundance of mosquitoes (t-value=-1.288; p-value= 0.23865; t-value= -0.385; p-value= 0.71139, respectively), and did not influence on the abundance of mosquitoes.
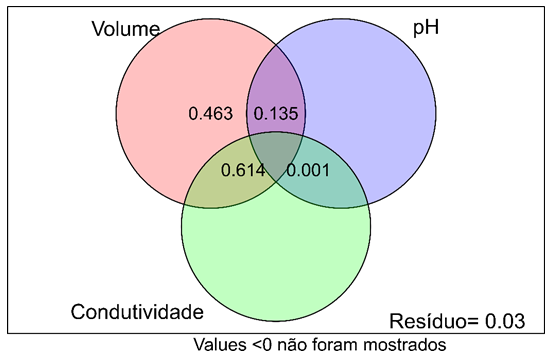
The environmental variables tested explained 97.3% of all the variation in the Culicidae community in bromeliads, indicating that the patterns of the larval composition of mosquitoes are greatly influenced by the environmental variables studied. The variance partition analysis showed that the water volume in the bromeliads provided an isolated explanation of 46.3 % for the mosquito species composition (Figure 3).
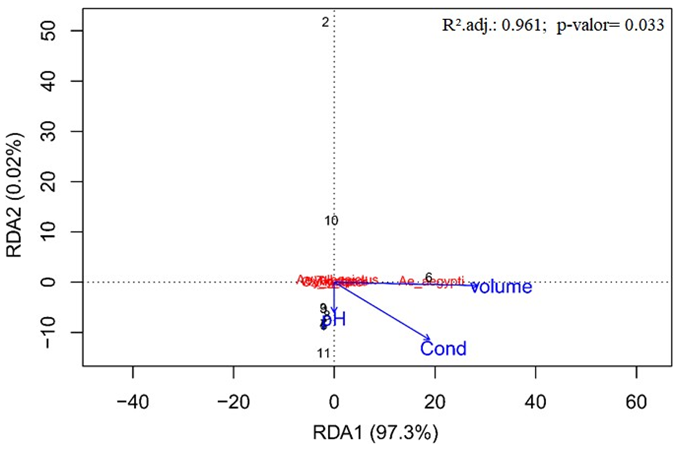
A positive relationship between the abundance of Aedes aegypti and bromeliads that have large volumes of water was also observed, as shown in the diagram in Figure 4.
The indices of ovitrap positivity and egg density are shown in table 01. The presence of Ae. aegypti was observed in both environments, but with a much higher prevalence in the urbanized environment. Table 01. Ovitrap positivity index (OPI) and egg density index (EDI) obtained during five consecutive weeks in rural and urban areas in the city of Londrina, PR.
Table 1: Ovitrap positivity index (OPI) and egg density index (EDI) obtained during five consecutive weeks in rural and urban areas in the city of Londrina, PR.
|
Rural |
Urban |
|||||
|
Weeks |
OPI (%) |
EDI |
Mean |
OPI (%) |
EDI |
Mean |
|
1 |
0 |
0 |
0 |
100 |
87.8 |
87.8 |
|
2 |
0 |
0 |
0 |
100 |
168 |
168 |
|
3 |
40 |
2.5 |
1 |
100 |
153.4 |
153.4 |
|
4 |
40 |
62.5 |
25 |
100 |
159.8 |
159.8 |
|
5 |
60 |
4 |
2.4 |
100 |
131.6 |
131.6 |
|
Total |
28 |
20.29 |
5.68 b* |
100 |
140.12 |
140.12 a |
*Means followed by different letters differ from each other by Tukey's test at the 5% level (p=0.0003; F=44.46).
4. Discussion
Brazil experiences dengue outbreaks seasonally every year, mainly in spring and summer in colder regions. Currently, 757.068 cases have been recorded up to the eighteenth epidemiological week, covering the interval between January and May 2022. The South and Southeast regions of the country stand out as the areas with the highest occurrence of cases, with rates of 1.171 cases/100.000 inhabitants and 635.6 cases/100 thousand inhabitants, respectively. Some works indicate that these plants are potential breeding grounds for Aedes [7-11], studying ornamental tank bromeliads grown in homes and public gardens at 51 sites in Miami County, USA, suggested that ornamental bromeliads are contributing to the proliferation of Ae. aegypti, highlighting that tank bromeliads should be considered in future vector control strategies for dengue, zika, and other arboviruses [13]. A recent study in 65 urban municipal parks in the city of São Paulo, Brazil, detected those immature forms of Culicidae, such as Ae. albopictus and Ae. aegypti, are common in bromeliad reservoirs that vegetate in these environments [12]. On the other hand, some studies indicate that bromeliads are not the preferred foci of Aedes [16]. According to [14], the environment of tank bromeliads makes it difficult to establish potential invaders from surrounding freshwater habitats. The differences in pH between bromeliad species are due to the decomposition processes of the accumulated organic material through release of organic acids and carbon dioxide. [15] concluded that bromeliads are the least suitable environments for the development of Ae. aegypti when compared to artificial containers, due to their acidic conditions. In addition, according to [6], bromeliads form a complex scenario, in which their importance in the reproduction of arbovirus vectors and other pathogens can vary according to the location, habitats, vector species, human behavior, and climate. Environmental variables have been identified as potential predictors of the presence, richness, and abundance of vector and non-vector mosquitoes [28-31], and the volume of water in bromeliads was found to be the main variable explaining the presence of the dengue vector in the areas studied in Londrina.
According to [18], when studying bromeliads native to Brazil cultivated in the Jardim Botânico de Bauru, SP, the phytotelma did not constitute the foci of Ae. aegypti or Ae. albopictus, with the prevailing presence of Culex. The authors explained this result by the location of the Botanical Garden, far from residential areas. However, they warned that tank bromeliads grown in urban areas, owing to the reduction in artificial breeding sites, can serve as alternative breeding sites for Ae. aegypti or Ae. albopictus, and that there is a need for monitoring and entomological control in these plants. It is generally observed that studies carried out in urbanized environments tend to point to bromeliads as possible breeding grounds for Ae. aegypti, while reports of larvae collection in phytotelma in natural environments do not find this mosquito in abundance [32-34]. The data obtained by ovitraps in the two environments studied here indicate the presence of Ae. aegypti in both environments, however in the rural environment this mosquito did not use bromeliads as a breeding ground, unlike the urban environment where a great abundance of Aedes was observed, especially if the plants have a large volume of water. The use of vector indicators in the form of an index is commonly used in various studies (e.g., OPI, EDI, ILHH), targeting one of the stages of the mosquito life cycle - egg, larva, pupa, or adult [35-38]. In the present study, we identified a higher index of positive eggs and ovitramps in the urban area, emphasizing the relationship between the urbanized environment and the presence of Ae. aegypti and Ae. albopictus. It is worth mentioning that the ovitraps are the most sensitive method to detect the presence of Aedes [37,39,40]. In this way, it appears that bromeliads, in their natural environment, cannot be considered a breeding ground for the larvae of this mosquito, but in an urbanized environment these plants, with storage of a large volume of water, can present an important source for mosquito larvae. The importance of weekly monitoring of this phytotelma in synanthropic environments is highlighted, and if necessary, the use of mainly larvicidal products to control larvae selectively, such as Bacillus thuringiensis subesp. israelenses. Studies carried out by [41] and [42] applied biolarvicides to artificial breeding sites and under laboratory conditions to control potential disease vectors in peri-urban areas, highlighting the importance of vector control.
Acknowledgment
To the laboratory technicians (Aparecido de Souza and Edson Santana) for their assistance with field collections. To the State General and Medical Entomology Laboratory University of Londrina for research support.
References
- Benzing DH. Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, (2000).
- Gouda EJ, Butcher D. A list of accepted Bromeliaceae names. University Botanic Gardens, Utrecht (2022).
- Martinelli G, Vieira C.M, Gonzalez M, Leitman P, Piratininga A, Costa AFD, Forzza RC. Bromeliaceae da Mata Atlântica brasileira: lista de espécies, distribuição e conservação. Rodriguésia, 59 (2008): 209-258.
- Docile TN, Figueiró R, Honório NA, Baptista DF, Pereira G, Dos Santos JAA, Codeco CT. Frequency of Aedes sp. Linnaeus (Diptera: Culicidae) and associated entomofauna in bromeliads from a forest patch within a densely urbanized area. Neotropical entomology, 46 (2017): 613-621.
- Paula CC. Cultivo prático de bromélias. 3ª ed. Viçosa: UFV, (2004) 106p.
- Frank JH, Lounibos LP. Insects and allies associated with bromeliads: a review. Terrestrial Arthropod Reviews 1 (2009): 125-153.
- Natal D, Urbinatti PR, Taipe-Lagos CB, Cereti-Júnior W, Diederichsen AT, Souza RG, Souza RP. Encontro de Aedes (Stegomyia) albopictus (Skuse) em Bromeliaceae na periferia de São Paulo, SP, Brasil. Revista de Saúde Pública, 31 (1997): 517-518.
- Forattini OP, Marques GRAM, Kakitani I, Brito, Sallum MAM. Significado epidemiológico dos criadouros de Aedes albopictus em bromélias. Saúde Pública 32 (1998): 186-188.
- Forattini OP, Marques GRAM. Nota sobre o encontro de Aedes aegypti em bromélias. Saúde Pública 34 (2000): 543-544.
- Marques GRAM, Santos RLC, Forattini OP. Aedes albopictus em bromélias de ambiente antrópico no Estado de São Paulo, Brasil. Saúde Pública 35 (2001): 243-248.
- Cunha SP, Alves JRC, Lima MM, Duarte JR, de Barros LC, da Silva JL, et al. Presença de Aedes aegypti em Bromeliaceae e depósitos com plantas no Município do Rio de Janeiro, RJ. Rev. de Saúde Pública. 36 (2002): 244-245.
- Ceretti-Junior W, Oliveira Christe R, Rizzo M, Strobel RC, de Matos Junior MO, Mello MHSH, et al. Species composition and ecological aspects of immature mosquitoes (Diptera: Culicidae) in bromeliads in urban parks in the city of Sao Paulo, Brazil. J. Arthropod. Borne Dis 10 (2016): 102-112.
- Wilke AB, Vasquez C, Mauriello PJ, Beier JC. Ornamental bromeliads of Miami-Dade County, Florida are important breeding sites for Aedes aegypti (Diptera: Culicidae). Parasites & Vectors 11 (2018): 283.
- Ospina-Bautista F, Varón JVE, Realpe E, Gast F. Diversidad de invertebrados acuáticos asociados a Bromeliaceae en un bosque de montaña. Rev. Colomb. Entomol 34 (2008): 224-229.
- Lopez LCS, Alves RRDN, Rios RI. Micro-environmental factors and the endemism of bromeliad aquatic fauna. Hydrobiologia 625 (2009): 151-156.
- Mocellin MG. Avaliação da importância das bromeliáceas como criadouro de Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera: Culicidae) no ambiente urbano do Rio de Janeiro. Tese (Doutorado em Biologia Parasitária), Instituto Oswaldo Cruz (2010).
- Santos CB, Leite GR, Falqueto A. Does native bromeliads represent important breeding sites for Aedes aegypti (L.) (Diptera: Culicidae) in urbanized areas. Neotropical Entomology, 40 (2011): 278-281.
- Oliveira VCD, Almeida Neto LCD. Ocorrência de Aedes aegypti e Aedes albopictus em bromélias cultivadas no Jardim Botânico Municipal de Bauru, São Paulo, Brasil. Saúde Pública 33 (2017): e00071016.
- Wrege MS, Steinmetz S, Reisser Júnior C, Almeida IR (Ed.). Atlas climático da Região Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul. Pelotas: Embrapa Clima Temperado; Colombo: Embrapa Florestas, (2011). 333 p.
- Legendre P, Legendre L. Numerical Ecology. Elsevier Science B.V. 2ª eds (1998): 853.
- Lourenço CB. O fitoplâncton na Zona Costeira Amazônica Brasileira: Biodiversidade, distribuição e estrutura no continuum estuário-oceano. Tese (Doutorado em Ciências), Centro de Energia Nuclear na Agricultura. Universidade de São Paulo (2016).
- Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 73 (1992): 1045-1055.
- Peres-Neto PR, Legendre P, Dray S, Borcard D. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87 (2006): 2614–2625.
- Oksanen J, Blanchet FG, Kindt R. R package version 1 (2011): 17-6.
- R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria (2020).
- Corrêa RR, Ramalho GR. Revisão de PhoniomyiaTheobaldi, 1903 (Diptera, Culicidae, Sabethini). Fiolia Clinica et Biológica 25 (1956): 1-176.
- Lopez LC, Silva EG, Beltrão MG, Leandro RS, Barbosa JE, Beserra EB. Effect of tank bromeliad micro-environment on Aedes aegypti larval mortality. Hydrobiologia 665 (2011): 257-261.
- Urbinatti PR, Menezes RMTD, Natal D. Sazonalidade de Aedes albopictus em área protegida na cidade de São Paulo, Brasil. Rev. Saúde Públ 41 (2007): 478-481.
- Arcos AN, Ferreira FAS, Cunha HB and Tadei WP. Characterization of artificial larval habitats of Anopheles darlingi (Diptera: Culicidae) in the Brazilian Central Amazon. Rev. Bra. Entomol 62 (2018): 267-274.
- Arcos AN, Valente-Neto F, Ferreira FAS, Bolzan FP, Cunha HB, Tadei WP, et al. Seasonality modulates the direct and indirect influences of forest cover on larval anopheline assemblages in western Amazônia. Scientific Reports 11 (2021): 12721.
- Arcos AN, da Silva Ferreira FA, Tadei WP, da Cunha HB, Roque RA. Limnological variables associated with the presence of Anopheles Meigen, 1818 (Diptera: Culicidae) larvae in breeding sites in Amazonas, Brazil. Revista Chilena de Entomología, 49 (2023).
- Varejão JBM, Santos CBD, Rezende HR, Bevilacqua LC, Falqueto A. Criadouros de Aedes (Stegomyia) aegypti (Linnaeus, 1762) em bromélias nativas na Cidade de Vitória, ES. Rev Soc Bra Med Trop 38 (2005): 238-240.
- Gonçalves KS, Messias MC. Ocorrência de Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Insecta, Diptera, Culicidae) em bromélias, no município do Rio de Janeiro (Rio de Janeiro, Brasil). Biota Neotrop 8 (2008): 235-237.
- Stein M, Juri MJD, Oria GI, Ramirez PG. Aechmea distichantha (Bromeliaceae) epiphytes, potential new habitat for Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) collected in the province of Tucumán, northwestern Argentina. Florida Entomologist 96 (2013): 1202-1206.
- Gomes AC. Medidas dos níveis de infestação urbana para Aedes (stegomya) aegypti e Aedes (stegomya) albopictus em programa de vigilância entomológica. Informe Epidemiológico SUS Brasília 7 (1998): 49-57.
- Tadei WP, Passos RA, Rodrigues IB, Santos JMM, Rafael MS. Indicadores entomológicos e o risco de transmissão de malária na área de abrangência do projeto PIATAM. In: Cavalcante, KV, Rivas AAF, Freitas CEC. (Eds.), Indicadores Socioambientais e Atributos de Referência para o trecho Urucu-Coari- Manaus, Rio Solimões, Amazônia 160 (2007).
- Depoli PAC, Zequi JAC, Nascimento KLC, Lopes J. Eficácia de Ovitrampas com Diferentes Atrativos na Vigilância e Controle de Aedes. EntomoBrasilis 9 (2016): 51–55.
- Zequi JAC, de Oliveira AA, dos Santos FP, Lopes J. Monitoramento e controle de Aedes aegypti (Linnaeus, 1762) e Aedes albopictus (Skuse, 1984) com uso de ovitrampas. Semin. Cienc Biol Saude 39 (2019): 93-102.
- Silva WR, Soares-da-Silva J, Ferreira FAS, Rodrigues IB, Tadei WP, Zequi JAC. Oviposition of Aedes aegypti Linnaeus, 1762 and Aedes albopictus Skuse, 1894 (Diptera: Culicidae) under laboratory and field conditions using ovitraps associated to different control agents, Manaus, Amazonas, Brazil. Rev Bra Entomol 62 (2018): 304-310.
- Silva KR, Ribeiro da Silva W, Silva BP, Arcos AN, Silva Ferreira FA, Soares- da-Silva J, et al. New traps for the capture of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse)(Diptera: Culicidae) eggs and adults. PLoS Neg. Trop. Dis 15 (2021): e0008813.
- Ferreira FADS, Arcos AN, Sampaio RTDM, Rodrigues IB, Tadei WP. Effect of Bacillus sphaericus Neide on Anopheles (Diptera: Culicidae) and associated insect fauna in fish ponds in the Amazon. Rev. Bra. Entomol. 59 (2015): 234-239.
- Soares-da-Silva J, Pinheiro VCS, Litaiff-Abreu E, Polanczyk RA, Tadei WP. Isolation of Bacillus thuringiensis from the state of Amazonas, in Brazil, and screening against Aedes aegypti (Diptera, Culicidae). Rev. Bra. Entomol 59 (2015): 01-06.



 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
