Thermobalancing therapy with Dr Allen's Device dissolves kidney stones improving health-related quality of life measured by the Wisconsin Stone Quality of Life questionnaire (WISQOL) – a randomised clinical trial
Article Information
Simon Allena, Muhammad Akramb, Ariana Adjania, Shahid Kamalb, Abid Rashidb, Akram Malikc, Muhammad Talha Khalilb, Sherwani Rehand
Affiliation:
aFine Treatment, Oxford, United Kingdom
bGovernment College University Faisalabad,
Pakistan
cMadinah Teaching Hospital, Faisalabad, Pakistan
dUniversity of Punjab Lahore, Pakistan
*Corresponding author:
Simon Allen, MD, PhD. Director and Head of Medical Research, Fine Treatment, Oxford, United Kingdom.
Received:March 20, 2022; Accepted:March 27, 2023;Published: April 05, 2023
Citation: Simon Allen, Muhammad Akram, Ariana Adjani, Shahid Kamal, Abid Rashid, Akram Malik, Muhammad Talha Khalil, Sherwani Rehan. Thermobalancing therapy with Dr Allen's Device dissolves kidney stones improving health-related quality of life measured by the Wisconsin Stone Quality of Life questionnaire (WISQOL) – a randomised clinical trial. Archives of Nephrology and Urology. 6 (2023): 14-22.
View / Download Pdf Share at FacebookAbstract
Introduction: Kidney stone disease (KSD) is associated with recurring pain, renal colic, and other debilitating symptoms, decreasing healthrelated quality of life (HRQoL) in patients.
Objective: The purpose of this study is to investigate the efficacy of Dr Allen’s Device and Thermobalancing therapy in dissolving kidney stones and in improving the quality of life in patients with nephrolithiasis.
Methods: A randomised, interventional, parallel group clinical trial investigated 54 patients aged 18-60 years with nephrolithiasis. 29 of the 54 patients with kidney stones under 10 mm were randomised (1:1) into a treatment group of 15 patients (Group 1) and a control group of 14 patients. The remaining 25 patients (Group 2) were treated but not randomised as they had stones larger than 10 mm. Both treatment groups used Dr Allen’s Device for 6 months. Changes in the size of kidney stones were recorded using ultrasound. Patients’ quality of life was measured using the Wisconsin Stone Quality of Life Questionnaire (WISQoL) at regular intervals and compared between the groups.
Results: Dr Allen's Device showed a significant improvement in HRQoL across all domains of the WISQoL and a significant reduction in the size of kidney stones in both treatment groups (p<0.001). There was no significant change in the stone size in the control group, and HRQoL improved only slightly across WISQoL domains. No side effects or complications were reported by patients in both treatment groups during the 6-month period.
Conclusions: The use of Thermobalancing therapy with Dr Allen's Device for Kidney Treatment is effective in dissolving kidney stones and in improving the quality of life of patients. This out-of-hospital treatment dissolves kidney stones of various composition non-invasively and without adverse events. Thus, Dr Allen’s Device is an effective and safe at-home treatment option for kidney stone disease.
Keywords
Kidney stone disease (KSD); Nephrolithiasis; Urolithiasis; Kidney stones; Thermobalancing therapy; Dr Allen's Device for Kidney Treatment; Wisconsin Stone Quality of Life Questionnaire (WISQoL); Health-related quality of life (HRQoL)
Kidney stone disease (KSD) articles; Nephrolithiasis articles; Urolithiasis articles; Kidney stones articles; Thermobalancing therapy articles; Dr Allen's Device for Kidney Treatment articles; Wisconsin Stone Quality of Life Questionnaire (WISQoL) articles; Health-related quality of life (HRQoL) articles
Kidney stone disease articles Kidney stone disease Research articles Kidney stone disease review articles Kidney stone disease PubMed articles Kidney stone disease PubMed Central articles Kidney stone disease 2023 articles Kidney stone disease 2024 articles Kidney stone disease Scopus articles Kidney stone disease impact factor journals Kidney stone disease Scopus journals Kidney stone disease PubMed journals Kidney stone disease medical journals Kidney stone disease free journals Kidney stone disease best journals Kidney stone disease top journals Kidney stone disease free medical journals Kidney stone disease famous journals Kidney stone disease Google Scholar indexed journals Nephrolithiasis articles Nephrolithiasis Research articles Nephrolithiasis review articles Nephrolithiasis PubMed articles Nephrolithiasis PubMed Central articles Nephrolithiasis 2023 articles Nephrolithiasis 2024 articles Nephrolithiasis Scopus articles Nephrolithiasis impact factor journals Nephrolithiasis Scopus journals Nephrolithiasis PubMed journals Nephrolithiasis medical journals Nephrolithiasis free journals Nephrolithiasis best journals Nephrolithiasis top journals Nephrolithiasis free medical journals Nephrolithiasis famous journals Nephrolithiasis Google Scholar indexed journals Urolithiasis articles Urolithiasis Research articles Urolithiasis review articles Urolithiasis PubMed articles Urolithiasis PubMed Central articles Urolithiasis 2023 articles Urolithiasis 2024 articles Urolithiasis Scopus articles Urolithiasis impact factor journals Urolithiasis Scopus journals Urolithiasis PubMed journals Urolithiasis medical journals Urolithiasis free journals Urolithiasis best journals Urolithiasis top journals Urolithiasis free medical journals Urolithiasis famous journals Urolithiasis Google Scholar indexed journals Kidney stones articles Kidney stones Research articles Kidney stones review articles Kidney stones PubMed articles Kidney stones PubMed Central articles Kidney stones 2023 articles Kidney stones 2024 articles Kidney stones Scopus articles Kidney stones impact factor journals Kidney stones Scopus journals Kidney stones PubMed journals Kidney stones medical journals Kidney stones free journals Kidney stones best journals Kidney stones top journals Kidney stones free medical journals Kidney stones famous journals Kidney stones Google Scholar indexed journals Thermobalancing therapy articles Thermobalancing therapy Research articles Thermobalancing therapy review articles Thermobalancing therapy PubMed articles Thermobalancing therapy PubMed Central articles Thermobalancing therapy 2023 articles Thermobalancing therapy 2024 articles Thermobalancing therapy Scopus articles Thermobalancing therapy impact factor journals Thermobalancing therapy Scopus journals Thermobalancing therapy PubMed journals Thermobalancing therapy medical journals Thermobalancing therapy free journals Thermobalancing therapy best journals Thermobalancing therapy top journals Thermobalancing therapy free medical journals Thermobalancing therapy famous journals Thermobalancing therapy Google Scholar indexed journals Dr Allen's Device for Kidney Treatment articles Dr Allen's Device for Kidney Treatment Research articles Dr Allen's Device for Kidney Treatment review articles Dr Allen's Device for Kidney Treatment PubMed articles Dr Allen's Device for Kidney Treatment PubMed Central articles Dr Allen's Device for Kidney Treatment 2023 articles Dr Allen's Device for Kidney Treatment 2024 articles Dr Allen's Device for Kidney Treatment Scopus articles Dr Allen's Device for Kidney Treatment impact factor journals Dr Allen's Device for Kidney Treatment Scopus journals Dr Allen's Device for Kidney Treatment PubMed journals Dr Allen's Device for Kidney Treatment medical journals Dr Allen's Device for Kidney Treatment free journals Dr Allen's Device for Kidney Treatment best journals Dr Allen's Device for Kidney Treatment top journals Dr Allen's Device for Kidney Treatment free medical journals Dr Allen's Device for Kidney Treatment famous journals Dr Allen's Device for Kidney Treatment Google Scholar indexed journals Wisconsin Stone Quality of Life Questionnaire articles Wisconsin Stone Quality of Life Questionnaire Research articles Wisconsin Stone Quality of Life Questionnaire review articles Wisconsin Stone Quality of Life Questionnaire PubMed articles Wisconsin Stone Quality of Life Questionnaire PubMed Central articles Wisconsin Stone Quality of Life Questionnaire 2023 articles Wisconsin Stone Quality of Life Questionnaire 2024 articles Wisconsin Stone Quality of Life Questionnaire Scopus articles Wisconsin Stone Quality of Life Questionnaire impact factor journals Wisconsin Stone Quality of Life Questionnaire Scopus journals Wisconsin Stone Quality of Life Questionnaire PubMed journals Wisconsin Stone Quality of Life Questionnaire medical journals Wisconsin Stone Quality of Life Questionnaire free journals Wisconsin Stone Quality of Life Questionnaire best journals Wisconsin Stone Quality of Life Questionnaire top journals Wisconsin Stone Quality of Life Questionnaire free medical journals Wisconsin Stone Quality of Life Questionnaire famous journals Wisconsin Stone Quality of Life Questionnaire Google Scholar indexed journals Health-related quality of life articles Health-related quality of life Research articles Health-related quality of life review articles Health-related quality of life PubMed articles Health-related quality of life PubMed Central articles Health-related quality of life 2023 articles Health-related quality of life 2024 articles Health-related quality of life Scopus articles Health-related quality of life impact factor journals Health-related quality of life Scopus journals Health-related quality of life PubMed journals Health-related quality of life medical journals Health-related quality of life free journals Health-related quality of life best journals Health-related quality of life top journals Health-related quality of life free medical journals Health-related quality of life famous journals Health-related quality of life Google Scholar indexed journals extracorporeal shock wave lithotripsy articles extracorporeal shock wave lithotripsy Research articles extracorporeal shock wave lithotripsy review articles extracorporeal shock wave lithotripsy PubMed articles extracorporeal shock wave lithotripsy PubMed Central articles extracorporeal shock wave lithotripsy 2023 articles extracorporeal shock wave lithotripsy 2024 articles extracorporeal shock wave lithotripsy Scopus articles extracorporeal shock wave lithotripsy impact factor journals extracorporeal shock wave lithotripsy Scopus journals extracorporeal shock wave lithotripsy PubMed journals extracorporeal shock wave lithotripsy medical journals extracorporeal shock wave lithotripsy free journals extracorporeal shock wave lithotripsy best journals extracorporeal shock wave lithotripsy top journals extracorporeal shock wave lithotripsy free medical journals extracorporeal shock wave lithotripsy famous journals extracorporeal shock wave lithotripsy Google Scholar indexed journals percutaneous nephrolithotomy articles percutaneous nephrolithotomy Research articles percutaneous nephrolithotomy review articles percutaneous nephrolithotomy PubMed articles percutaneous nephrolithotomy PubMed Central articles percutaneous nephrolithotomy 2023 articles percutaneous nephrolithotomy 2024 articles percutaneous nephrolithotomy Scopus articles percutaneous nephrolithotomy impact factor journals percutaneous nephrolithotomy Scopus journals percutaneous nephrolithotomy PubMed journals percutaneous nephrolithotomy medical journals percutaneous nephrolithotomy free journals percutaneous nephrolithotomy best journals percutaneous nephrolithotomy top journals percutaneous nephrolithotomy free medical journals percutaneous nephrolithotomy famous journals percutaneous nephrolithotomy Google Scholar indexed journalsArticle Details
Introduction
Health-related quality of life (HRQoL) is reduced in patients with urolithiasis, especially during acute urolithiasis [1]. The Wisconsin Stone
Quality of Life Questionnaire (WISQoL) is the validated HRQoL instrument specific for nephrolithiasis and can be used to evaluate the quality of life in patients treated with different treatment modalities [2, 3].
Despite the high prevalence of urolithiasis, only recently has the WISQOL questionnaire appeared to assess the quality of life in these patients [4]. The suitability of this questionnaire in patients with urolithiasis was demonstrated in 2017 during a multi-centric study performed in 8 geographically diverse centres in the United States and Canada [5].
According to clinical guidelines, in cases of symptomatic urolithiasis, extracorporeal shock wave lithotripsy (ESWL), retrograde intrarenal surgery (RIRS) and percutaneous nephrolithotomy (PCNL) are offered as first-line therapy [6]. The effectiveness of surgical techniques is indicated by the so called “stone-free rate” [7, 8]. The percentage of complications after ESWL, PCNL, and RIRS was 15.4%, 13.8% and 18.3% respectively [9]. Almost 40% of first-time stone formers will have recurrence within 3 years of the first episode [10]. Thus, 42.7% of patients had stone recurrence, more often in men (44.4%) than in women (38.9%) [11].
The innovative medical technology utilising body heat in the management of chronic diseases, including urolithiasis, using therapeutic Dr Allen’s Device has been assessed in the International Journal of Quality Innovation [12]. Thermobalancing therapy and Dr Allen's Device were patented for their novelty [13]. Two clinical studies conducted on Thermobalancing therapy and Dr Allen’s Device in men with benign prostate enlargement (BPE) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) demonstrated efficacy and safety of this out-of-hospital treatment [14, 15].
The objectives of this clinical trial included:
- Studying the efficacy of Thermobalancing therapy in patients with kidney stones < 10 mm
- Studying the efficacy of Thermobalancing therapy in patients with kidney stones > 10 mm
The present study aimed to use the WISQoL questionnaire to assess the impact of Dr Allen's Device for Kidney Treatment on the HRQoL in patients with nephrolithiasis.
Materials and Methods
Study design and participants
Our study was a prospective, randomised, interventional, parallel group clinical trial conducted to demonstrate safety, efficacy, and advantages of treating patients with non- invasive Dr Allen’s Device. It was conducted between 2021- 2022 at the Government College University Faisalabad. This study on Thermobalancing therapy and Dr Allen’s Device was approved by the Ethics Review Committee of the Government College University Faisalabad.
Patients were recruited from Madinah Teaching Hospital, Faisalabad, Surraya Majeed Hospital, Faisalabad, and Al- Rashid Medicare, Faisalabad. All of them were treated at the Government College University Faisalabad.
Patients aged 18-60 years having a kidney stone measuring at least 3 mm in size confirmed by ultrasonography were recruited for the trial, as using the sound waves of an ultrasound to detect a painful kidney stone is just as effective as the X-rays [16]. They were excluded if they had severe co- morbidities such as cancer, heart failure or infection.
A total of 54 patients with urolithiasis met the eligibility criteria and were recruited for the study. The decision to randomise only those patients with kidney stones smaller than 10 mm was taken to avoid any serious consequences of leaving large kidney stones untreated for the period of the clinical trial. Patients with kidney stones less than 10 mm (n = 29) were randomised into two groups (1:1). 14 patients were randomly assigned to the control group and 15 were assigned to the treatment group (Group 1) using simple randomisation procedures and computer-generated random numbers. The remaining 25 patients with kidney stones greater than 10 mm in size were assigned to a separate treatment group (Group 2).
No blinding was done due to the nature of the intervention. Written consent was obtained from all participants of the study.
Sample size estimation
Sample size was calculated using G*Power software. With a power of 80% and a type I error of 5%, it was found that a total of 54 patients were required to conduct the study.
Intervention
The intervention was Thermobalancing therapy using Dr Allen's Device for a 6-month period as monotherapy. No pharmacological therapy was used in treatment groups.
Dr Allen's Device for Kidney Treatment
Dr Allen’s Device was registered with the Medicines and Healthcare Products Regulatory Agency in the United Kingdom in 2010 as a Class I medical device. The thermoelements in Dr Allen’s Device are made from a special wax-based material and need to be applied topically to the skin over the affected organs. In people with KSD, 2 thermoelements in Dr Allen’s Device are applied to the back over the kidney area (Figure 1). These thermoelements accumulate naturally emitted body heat and turn into a source of energy, directing this heat towards the kidneys.
In order to understand the effectiveness of this therapy on urolithiasis and particularly, on the HRQoL of the patients, the WISQoL was presented to the participants of the treatment groups as physical copies at the beginning of the study, at 2 weeks and then once each month for the duration of the trial and at the end; therefore, they were assessed a total of
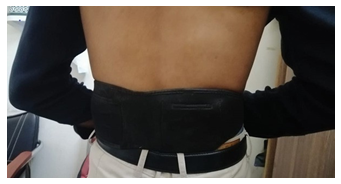
Figure 1: Dr Allen's Device for Kidney Treatment is applied to the back in projection of the kidneys
8 times. The participants of the control group were assessed 3 times, that is at the beginning of the trial, at 2 weeks and at the end of 6 months. Also measured by ultrasonography was the size of the stones at the beginning of the trial; it was repeated at the end of 6 months to calculate the change in the size of kidney stones. The composition of kidney stones was determined by examining patients’ urine for the presence of certain urine crystals.
Statistical analysis
The data collected was organised in Microsoft Excel and statistical analysis of the results was performed using the PAST software and the Data Analysis module of Microsoft Excel 2010. Paired sample t-tests were used for the comparison of continuous data. A p-value < 0.05 was considered statistically significant.
Results
After screening 57 patients, 3 patients were excluded as they did not meet eligibility criteria. Out of the remaining 54 patients, 25 were excluded from randomisation as they had kidney stones greater than 10 mm in size and, therefore, could not be allocated to the control group in view of potential risks associated with untreated large kidney stones. 29 patients were randomised, resulting in two groups. The treatment group consisted of 15 patients (Group 1) and the control group consisted of 14 patients. The 25 patients with stones larger than 10 mm in size were grouped together and assessed as a separate treatment group (Group 2).
The clinical characteristics of patients in the two treatment groups and the control group were similar and are presented in Table 1. The flow diagram is shown in Table 2.
The sizes of kidney stones were measured in all participants at the beginning and the end of the clinical trial. Figures 2 and 3 summarise the change in the sizes of the kidney stones in Group 1 and Group 2 respectively.
In the treatment Group 1, out of 15 patients with stone size under 10 mm, 12 patients had stones in the left kidney and 9 patients had stones in the right kidney, meaning that 6 patients had stones bilaterally, and a total of 21 stones were treated. After the use of Dr Allen's Device for 6 months, 6 of the 12 patients with stones in the left kidney dissolved their stones completely, while the remaining 6 patients had the stones significantly reduced to 3 mm. Similarly, after the use of Dr Allen's Device for 6 months, 5 of the 9 patients with stones in the right kidney dissolved their stones completely, while the remaining 4 patients had the stones significantly reduced to 3 mm. Thus, out of 21 stones treated, the 6-month use of Dr Allen’s Device dissolved 11 kidney stones completely, while the remaining 10 kidney stones were significantly reduced to 3 mm, (p<0.001), Figure 2.
In the treatment Group 2, out of 25 patients with stone size larger than 10 mm, 14 patients had stones in the left kidney and 19 patients had stones in the right kidney, meaning that 8 patients had stones bilaterally, and a total of 33 stones were treated. After the use of Dr Allen's Device for 6 months, the size of the stones reduced in 19 patients, (p<0.005). By the end of the treatment period, out of these 33 large stones, 9 stones still measured over 10 mm, while 24 stones were reduced to 10 mm or less, Figure 3.
In the control group, there was either no change in the size of kidney stones in either kidney or an increase in size. Thus, in the control group, the size of stones in the right kidney increased from 4.8 ± 3.4 to 5.3 ± 3.6, p=0.73, while the size of stones in the left kidney increased from 4.3 ± 3.1 to 4.7 ± 3.6, p=0.78.
Symptom progression affecting the HRQoL was measured using the domains of the WISQoL: Social Influence (SI), Emotional Influence (EI), Health Effect (HE), and Influence on Vital Activity (IVA). Patients in treatment groups were assessed a total of 8 times. The two treatment groups were investigated separately. The average scores of the 4 domains of the WISQoL in the treatment groups are presented in Figures 4 and 5.
Table 1: Clinical characteristics of the 54 patients with KSD
|
Group 1 |
Group 2 |
Control Group |
|
|
Number of patients (n) |
15 |
25 |
14 |
|
Mean age in years |
38.6 |
38.5 |
39.8 |
|
Gender (Male:Female) |
4:11 |
12:13 |
13:1 |
|
Location of stone (n): |
|||
|
Left kidney |
9 |
14 |
10 |
|
Right kidney |
12 |
19 |
11 |
|
Bilateral kidney stones |
6 |
8 |
7 |
|
Composition of stone (n): |
|||
|
Calcium oxalate stones |
11 |
12 |
11 |
|
Uric acid stones |
1 |
8 |
2 |
|
Triple phosphate stones |
3 |
5 |
1 |
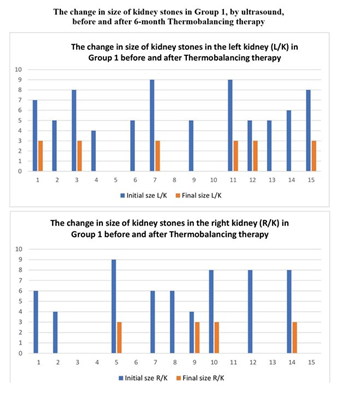
Figure 2: The change in the size of kidney stones in Group 1 measured by ultrasound before and after the 6-month treatment period with Thermobalancing therapy and Dr Allen’s Device in Left and Right kidneys
In the 15 patients in Group 1, the WISQoL scores increased in all domains. The mean Social Influence (SI) score increased slightly in 2 weeks from 9.47 ± 4.79 to 10.27 ± 4.03 and significantly to 16.67 ± 1.45 in next 5-6 months (p<0.001). The Emotional Influence (EI) score increased slightly in 2 weeks from 8.6 ± 4.88 to 12.67 ± 4.81 and significantly to 21.3 ± 2.35 in the next 5-6 months (p<0.001). The Health Effect (HE) score increased slightly in 2 weeks from 12.13 ± 3.14 to 16 ± 5.74 and significantly to 26.4 ± 2.13 in the next 5-6 months (p<0.001). The Influence on Vital Activity (IVA) score increased slightly in 2 weeks from 3.8 ± 1.57 to 7.47 ± 2.90 and significantly to 12.67 ± 1.54 in the next 5-6 months (p<0.001). The total score increased slightly in 2 weeks from 34.0 ± 9.15 to 46.4 ± 16.49 and significantly to 77.07 ± 7 in the next 5-6 months P<0.001), Figure 4.
The control group showed improved scores across all domains but the increase was only significant in SI and HE. The mean SI score increased from 9.43 ± 4.88 to 12.43 ±
3.27 in 6 months (p = 0.03) and the mean HE score increased from 14.00 ± 5.43 to 17.29 ± 3.67 in 6 months (p = 0.03). The p-values for mean EI and IVA were 0.22 and 0.1 respectively. The total score increased only slightly during the 6-month period from 43.13 ± 17.33 to 53.14 ± 9.46 (p = 0.04).
In the 25 patients in Group 2, the WISQoL scores also increased in all domains. The mean SI score decreased slightly in 2 weeks from 11.24 ± 5.45 to 10.6 ± 3.33 and increased significantly to 16.32 ± 1.75 by the end of 6 months (p = 0.020). The mean EI score increased slightly in 2 weeks from 9.0 ± 5.68 to 14.08 ± 4.71 and significantly to 20.88 ± 1.81 by the next 6 months (p<0.001). The mean HE score increased slightly in 2 weeks from 15.48 ± 4.54 to 17.08 ± 6.26 and significantly to 26.40 ± 1.81 in the next 6 months (p<0.001). The mean IVA score increased slightly in 2 weeks from 4.88 ± 3.05 to 8.12 ± 2.12 and significantly to 12.60 ± 1.22 in the next 6 months (p<0.001). The total score increased slightly in 2 weeks from 40.60 ± 14.22 to 49.88 ± 16.67 and significantly to 76.20 ± 5.56 in the next 6 months (p<0.001), Figure 5.
A comparison of the improvement across all domains of the WISQoL is demonstrated in Table 3.
The presented results of the dynamics of WISQoL indicators demonstrate that Dr Allen's Device significantly improves the quality of life of patients with urolithiasis starting from 2 weeks of wearing the device and in the following 6 months.
Harms: The use of Dr Allen’s Device did not cause any adverse events in all participants. Neither pain, nor renal colic were reported in the treatment groups during the study period. No pharmacological intervention was required.
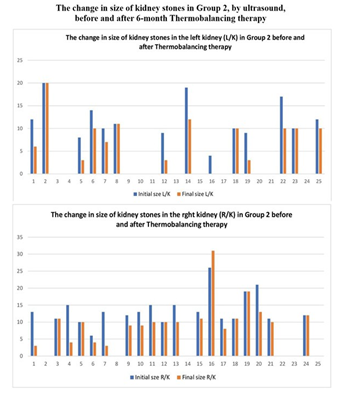
Figure 3: The change in the size of kidney stones in Group 2 measured by ultrasound before and after 6-month treatment period with Thermobalancing therapy and Dr Allen’s Device in Left and Right kidneys
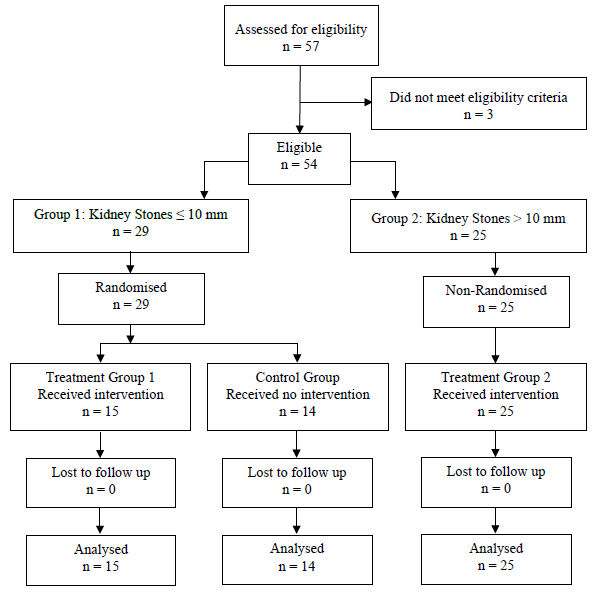
Table 2: Patient allocation flow diagram.
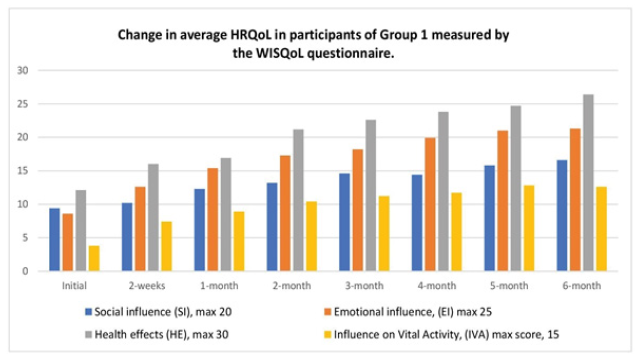
Figure 4: The change in HRQoL measured by the WISQOL questionnaire in Group 1 during the 6-month treatment period with Thermobalancing therapy and Dr Allen’s Device
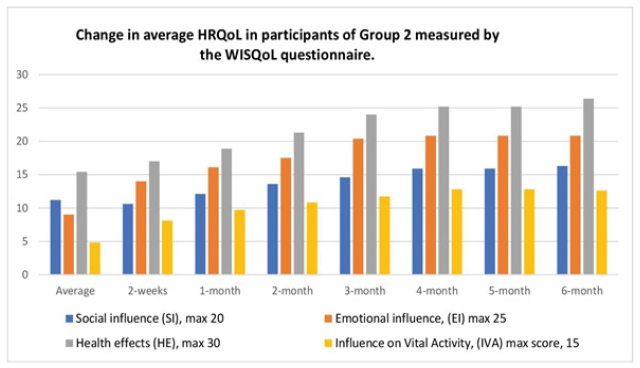
Figure 5: The change in HRQoL measured by the WISQoL questionnaire in Group 2 during the 6-month treatment period with Thermobalancing therapy and Dr Allen’s Device
Table 3: Comparison of the scores of all domains of WISQoL between the control group and treatment groups
|
Initial evaluation |
Final evaluation |
p-value |
||
|
(mean ± SD in mm) |
(mean ± SD in mm) |
|||
|
Control Group |
SI |
9.43 ± 4.88 |
12.43 ± 3.27 |
0.03 |
|
EI |
12.79 ± 5.44 |
14.43 ± 2.98 |
0.22 |
|
|
HE |
14.00 ± 5.43 |
17.29 ± 3.67 |
0.03 |
|
|
IVA |
7.36 ± 3.56 |
9.00 ± 2.04 |
0.1 |
|
|
Total Score |
43.13 ± 17.33 |
53.14 ± 9.46 |
0.04 |
|
|
Group 1 |
SI |
9.47 ± 4.79 |
16.67 ± 1.45 |
<0.001 |
|
EI |
8.6 ± 4.88 |
21.33 ± 2.35 |
<0.001 |
|
|
HE |
12.13 ± 3.14 |
26.4 ± 2.13 |
<0.001 |
|
|
IVA |
3.8 ± 1.57 |
12.67 ± 1.54 |
<0.001 |
|
|
Total Score |
34 ± 9.15 |
77.07 ± 7 |
<0.001 |
|
|
Group 2 |
SI |
11.24 ± 5.45 |
16.32 ± 1.75 |
<0.001 |
|
EI |
9.00 ± 5.68 |
20.88 ± 1.81 |
<0.001 |
|
|
HE |
15.48 ± 4.54 |
26.40 ± 1.96 |
<0.001 |
|
|
IVA |
4.88 ± 3.05 |
12.60 ± 1.22 |
<0.001 |
|
|
Total Score |
40.60 ± 14.22 |
76.20 ± 5.56 |
<0.001 |
|
|
Note. WISQoL domains: SI - social influence, EI - emotional influence, HE - health effect, IVA - influence on vital activity. |
||||
Discussion
For many years, it has been difficult to evaluate the effectiveness of kidney stone treatment, especially assessing long-term HRQoL of patients using the NIH-sponsored PROMIS-43 questionnaire or the Short-Form 36-item survey [17, 18]. Recently developed WISQoL scores have been found to have good predictive validity for HRQoL [19]. The use of the WISQoL questionnaire in different countries has proven that it is a consistent, reliable, and valid instrument to assess HRQoL in patients with symptomatic urolithiasis [20, 21]. In our study, changes in the quality of life of patients with urolithiasis were assessed using the WISQoL.
The results differed in patients with stones up to 10 mm in size (Group 1) and in patients with stones larger than 10 mm (Group 2). It was recorded that in Group 1, a significant increase in all domains of WISQoL was detected in the 2nd month of using Dr Allen Device. In Group 2, the rise in all domains developed gradually, with a significant increase in all domains of WISQoL occurring after 3 months. In both treatment groups, the numbers in all domains increased close to their maximum possible score at the end of treatment with Dr Allen’s Device.
This improvement in HRQoL correlated with a reduction in kidney stone size. In 15 patients in Group 1, Dr Allen’s Device reduced the size of kidney stones in all patients either down to 3 mm or dissolved the stones completely. Out of 25 patients in Group 2, the 6-month use of Dr Allen's Device reduced the size of kidney stones in 19 patients. Dr Allen's Device reduced the size of kidney stones regardless of their composition: calcium oxalate, uric acid, and triple phosphate, and thereby improved the HRQoL in patients.
Also, our empirical results support Allen’s theoretical framework of pathophysiology of the formation and growth of kidney stones in patients. In response to initial triggers, such as excess of crystals in the kidney tissue (calcium oxalate, uric acid, etc.), the constriction of capillaries occurs creating micro-focal hypothermia, i.e. the secondary trigger, that leads to the spontaneous expansion of the capillary net and, consequently, to increased pressure inside the kidney tissue. This process is responsible for the continuous formation of kidney stones, and the disease becomes chronic [22].
The same processes at the capillary level have been attributed to the development of chronic non-malignant prostate diseases. It was explained that the expansion of capillaries in the prostate, caused by micro-focal hypothermia as the secondary trigger, gradually formed additional tissue, creating pressure in the prostate gland making it to enlarge in size [23, 24]. In two previous clinical studies, Thermobalancing therapy with Dr Allen’s Device demonstrated a significant decrease in prostate volume and a reduction in urinary symptoms in men with prostate enlargement, and with chronic prostatitis by means of improving blood circulation in the prostate though temperature regulation [25]. Dr Allen's Device is shown to be an effective tool for the at-home management of chronic prostate diseases [26, 27].
Analogously, it can be explained that by means of continuous thermal exposure, not exceeding normal body temperature, thermobalancing therapy eliminates micro-focal hypothermia in the kidney tissue, i.e. the secondary trigger responsible for the formation of kidney stones, inhibiting spontaneous expansion of capillaries [22]. This study suggests that the use of Dr Allen's Device improves blood circulation in the tissue of the kidneys, which leads to a decrease in the size of kidney stones or their complete dissolution.
We recognise that the presence of a "placebo" group as control could have provided more statistical rigor concerning results. However, 6 months may be considered an appropriate time for oral medication as placebo, but not for using a medical device required to be worn on the body for most of the day for 6 months. Therefore, this clinical trial was conducted without the use of a placebo, and we consider this a limitation of our study.
This clinical trial shows the advantages of using Dr Allen’s Device over other standard treatment options for KSD. The initial promising results of ESWL were challenged by adverse effects such as bleeding, pain, and urinary tract infections, rare complications, and long-term consequences such as hypertension and diabetes mellitus [28-32]. PCNL is usually used for removing kidney stones over 2 cm in size, and may produce various complications, such as bleeding, extravasation or urine, pleural drain, renal embolization etc. [33, 34].
Considering the severe complications after PCNL, RIRS is sometimes suggested as a treatment alternative to PCNL, particularly in lower pole stones larger than 2 cm [35, 36]. However, the prevalence of infectious complications among patients undergoing RIRS for kidney stones is 7.7%, and it is impossible to predict which patients will develop these complications [37].
In contrast to these standard treatments, no side effects or complications were reported by patients using Dr Allen’s Device in both treatment groups during the 6-month period. This study, therefore, demonstrates that Dr Allen's Device is completely safe to use in all patients with kidney stones, small or large. Moreover, its use supports successful ageing in patients with kidney stones [38]. The improvement in the HRQoL with thermobalancing therapy was recorded in all patients, even when the change in the size of kidney stones was insignificant. This evidence demonstrates that Dr Allen's Device improves the overall health condition of patients with KSD.
Conclusion
The study demonstrates that, in most patients with nephrolithiasis, Dr Allen's Device for Kidney Treatment reduces the size of kidney stones or dissolves them completely. This reduction in the size of kidney stones is achieved regardless of their composition or initials size, small or large. Evidence suggests that the dissolving of kidney stones with thermobalancing therapy occurs as the result of the improvement of blood circulation in the kidney tissue under local temperature regulation. A significant improvement of the HRQoL in patients in both treatment groups was recorded across all domains of the WISQoL questionnaire. Changes in the control group were insignificant. No renal colic, pain, side effects, or complications were experienced by patients while using Dr Allen’s Device at home. The evidence demonstrates that Dr Allen's Device for Kidney Treatment is an effective and safe out-of-hospital solution for kidney stone disease.
Conflict of interest
The Authors declare that there is no conflict of interest.
Informed Consent
Written informed consent was obtained from all participants before the start of the study.
Availability of data and materials
The datasets used and/or analysed during the current study and the full trial protocol are available from the corresponding author upon reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
Financial support for this study has been obtained from the European Regional Development Fund (ERDF).
Acknowledgements
We are grateful to the Oxfordshire Local Enterprise Partnership (OxLEP) and the ERDF for their support in developing our medical innovation that advances the treatment of kidney stone disease and improves patient care.
References
- Ellison JS, Williams M, Keeley FX. Jr Patient-Reported Outcomes in Nephrolithiasis: Can We Do Better? J Endourol 32(1) (2018):10-20.
- Gottstein, , Pratsinis, M., Güsewell, S. et al The German linguistic validation of the Wisconsin Stone Quality of Life questionnaire (WisQoL). World J Urol 39 (2021): 2163-2168.
- Yoon YE, Cho Translation and Linguistic Validation of the Korean Version of the Wisconsin Stone Quality of Life Questionnaire. Int Neurourol J 24(1) (2020):77-83.
- Penniston KL, Nakada Development of an instrument to assess the health-related quality of life of kidney stone formers. J Urol 189(3) (2013): 921-930.
- Penniston KL, Antonelli JA, Viprakasit DP, et Validation and Reliability of the Wisconsin Stone Quality of Life Questionnaire. J Urol 197(5) (2017):1280-1288.
- Assimos D, Krambeck A, Miller NL et Surgical management of stones: American Urological Association/ Endourological Society Guideline, part II. J Urol 196 (2016): 1161.
- Kim CH, Chung DY, Rha KH, et al. Effectiveness of Percutaneous Nephrolithotomy, Retrograde Intrarenal Surgery, and Extracorporeal Shock Wave Lithotripsy for Treatment of Renal Stones: A Systematic Review and Meta-Analysis. Medicina (Kaunas) 57(1) (2020):
- Kokov D, Manka L, Beck A, et al. Only Size Matters in Stone Patients: Computed Tomography Controlled Stone- Free Rates after Mini-Percutaneous Urol Int 103(2) (2019): 166-171.
- Chung DY, Kang DH, Cho KS, et Comparison of stone-free rates following shock wave lithotripsy, percutaneous nephrolithotomy, and retrograde intrarenal surgery for treatment of renal stones: A systematic review and network meta-analysis. PLoS One 14(2) (2019): e0211316.
- Johri N, Cooper B, Robertson W, et al. An update and practical guide to renal stone management. Nephron Clin Pract 116(3) (2010): c159-71.
- Daudon M, Jungers P, Bazin D et Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis 46 (2018): 459-470.
- Allen, Innovative Thermobalancing therapy and Dr Allen’s Device for the first time employ body energy to treat chronic prostatic diseases effectively. Int J Qual Innov (2020) 6:2
- Allen S, Adjani Therapeutic Device and Method, United States Patent and Trademark Office. Patent No.: US 9,408,744 B2. (2016) Aug. 9, Accessed Mar. 19, 2022.
- Allen S, Aghajanyan IG. Benign Prostatic Hyperplasia Treatment with New Physiotherapeutic Device, Urol J 12(5) (2015): 2371-2376.
- Allen S, Aghajanyan Effect of thermobalancing therapy on chronic prostatitis and chronic pelvic pain syndrome, Journal of Clinical Urology 10(4) (2016):1-8.
- Smith-Bindman R, Aubin C, Bailitz J, et Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med 371(12) (2014): 1100-10.
- Patel N, Brown RD, Sarkissian C, et Quality of life and urolithiasis: the patient - reported outcomes measurement information system (PROMIS). Int Braz J Urol 43(5) (2017): 880-886.
- Arafa MA, Rabah DM. Study of quality of life and its determinants in patients after urinary stone Health Qual Life Outcomes 8 (2010):119.
- Polotti C, Tan B, Borglum N, et Relationship Between the Wisconsin Stone Quality of Life (WISQOL) and Preference-Based/Health Utility Measures of Health- Related Quality of Life (HRQoL) in Kidney Stone Patients. Urology 141 (2020): 33-38.
- Basulto-Martínez M, Olvera-Posada D, Velueta-Martínez IA, et al Quality of life in patients with kidney stones: translation and validation of the Spanish Wisconsin Stone Quality of Life Questionnaire. Urolithiasis 48 (2020): 419-424.
- Atalay HA, Ülker V, Canat L, et al. Validation of the Turkish version of the Wisconsin stone-quality of life Turk J Urol 245(2) (2018): 118-123.
- Allen Dr. Allen’s Therapeutic Devices Should be Implemented in the Healthcare System for the Treatment of Chronic Noncancerous Prostate and Kidney Diseases Saving People’s Well-Being and Money, Ann Mil Health Sci Res. 2018; 16(2): e81033.
- Allen S, Aghajanyan Use of thermobalancing therapy in ageing male with benign prostatic hyperplasia with a focus on etiology and pathophysiology. Aging Male 14 (2016): 1-5.
- Allen S. The vascular factor plays the main role in the cause of pain in men with chronic prostatitis and chronic pelvic pain syndrome: the results of clinical trial on thermobalancing Diseases 5 (2017): 25.
- Aghajanyan IG, Allen Positive Response to Thermobalancing Therapy Enabled by Therapeutic Device in Men with Non-Malignant Prostate Diseases: BPH and Chronic Prostatitis. Diseases. 2016; 4(2):18.
- Allen Personalized care using thermobalancing therapy can help men with chronic prostatitis / chronic pelvic pain syndrome to recover, Personalized Medicine Universe. 2019, 8, 48-52.
- Adjani A, Allen The at-home delivery of treatment for benign prostate enlargement and chronic prostatitis enabled by Dr Allen’s Device is a valuable healthcare innovation during a pandemic. Medico Research Chronicles, 8(4) (2021): 334-344
- Skolarikos A, Alivizatos G, De la Rosette Extracorporeal shock wave lithotripsy 25 years later: complications and their prevention. European Urology 50(5) (2006): 981- 990.
- Marinkovic SP, Marinkovic CM, Xie D. Spleen injury following left extracorporeal shockwave lithotripsy (ESWL). BMC Urology 15 (2015): 4.
- Akbulut F, Kucuktopcu O, Ucpinar B, et A Rare Complication of Extracorporeal Shock Wave Lithotripsy: Intrarenal Hematoma Mimicking Pelvis Renalis Tumor. Case Rep Urol 2015 (2015): 719618.
- Krambeck AE, Gettman MT, Rohlinger AL et Diabetes Mellitus and Hypertension Associated with Shock Wave Lithotripsy of Renal and Proximal Ureteral Stones at 19 Years of Followup. J Urol 175(5) (2006):1742-1747.
- D’Addessi A, Vittori M, Racioppi M et Complications of Extracorporeal Shock Wave Lithotripsy for Urinary Stones: To Know and to Manage Them—A Review. Scientific World Journal 15 (2012): 619820.
- Fernström I, Johansson B Percutaneous pyelolithotomy. A new extraction Scand J Urol Nephrol 10(1976): 257-9.
- Michel MS, Trojan L, Rassweiler JJ. Complications in percutaneous nephrolithotomy. Eur Urol 51(4) (2007): 899-906.
- Sari S, Ozok HU, Cakici MC et Comparison of Retrograde Intrarenal Surgery and Percutaneous Nephrolithotomy for Management of Renal Stones ≥ 2 cm, Urology Journal 14(1) (2017): 2949-2954.
- Zengin K, Tanik S, Karakoyunlu N, et al. Retrograde Intrarenal Surgery versus Percutaneous Lithotripsy to Treat Renal Stones 2-3 cm in Diameter, BioMed Research International 10 (2015): 914231.
- Berardinelli F, De Francesco P, Marchioni M, et Infective complications after retrograde intrarenal surgery: a new standardized classification system. Int Urol Nephrol 48(11) (2016):1757-1762.
- Allen S, Adjani Benign Prostatic Hyperplasia and Kidney Stone Disease Thermobalancing Therapy with Dr. Allen’s Device: Key to Successful Ageing Without Medications, Surgery, and Risky Exposure to Coronavirus Infection, Nephro-Urol Mon. 2021 13(2): e110771.


 Impact Factor: * 2.3
Impact Factor: * 2.3 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 