Membranous Nephropathy- Over the Counter? NELL Yes!
Article Information
Umair Ali1, Neriman Gokden2, John Arthur1, Manisha Singh1*
1Department of Internal Medicine, Division of Nephrology, University of Arkansas for Medical Sciences, USA
2 Department of Pathology, University of Arkansas for Medical Sciences, USA
*Corresponding Author: Manisha Singh, Department of Internal Medicine, Division of Nephrology University of Arkansas for Medical Sciences, USA
Received: 23 November 2023; Accepted: 01 December 2023; Published: 28 March 2024
Citation: Umair Ali, Neriman Gokden, John Arthur, Manisha Singh. Membranous Nephropathy- Over the Counter? NELL Yes!. Archives of Nephrology and Urology. 7 (2024): 17-20.
View / Download Pdf Share at FacebookAbstract
In adults, membranous Nephropathy (MN) is a common cause of idiopathic nephrotic syndrome. Primary MN is caused by antibodies directed against podocyte antigens, while secondary MN with the deposition of circulating immune complexes in the same area of glomeruli. Most of the primary membranous are positive for PLA2R- and THSD7Aantibodies associated with the progression of the disease. Some novel antibodies like NELL 1 indicate primarily secondary causes, including malignancy but may sometimes present as a primary disease with no underlying reason or ingestion of lipoic acid. Our patient presented to the hospital with a rash and swelling of lower limbs, which was sudden in onset. With a history of diabetes mellitus with comorbidities, he used multiple medications, including over-the-counter supplements (lipoic acid). Renal biopsy revealed NELL 1 positivity likely secondary to overthe- counter supplement usage.
Keywords
Membranous Nephropathy (MN), Lipoic Acid (LA), Nephrotic Syndrome (NS), Renal Biopsy (RB), Renal Antigens (RA).
Article Details
1. Introduction
Membranous Nephropathy (MN) is the most common cause of nephrotic syndrome in predominantly white men [1]. MN is a chronic disease that may course to spontaneous remissions (usually within the first two years) or frequent relapses. The severe disease typically progresses to end-stage renal disease [1]. MN is presumed to be caused by the deposition of immune complexes with deposits against podocyte antigens or circulating immune complexes deposits [2]. MN can also be classified into primary (idiopathic) and secondary as in autoimmune diseases (membranous lupus nephritis), NSAIDs, infections (such as viral hepatitis), graft-versus-host disease, allergic responses (such as against bovine serum albumin) and medications [2]. An autoimmune response was first recognized in 1959, with the discovery of M-type phospholipase A2 receptor 1 (PLA2R) and thrombospondin type 1 domain-containing 7A (THSD7A). These were present in approximately 70% and 1%-5% of patients with primary MN, respectively [3]. Although these antigens are mostly related to primary MN. Sometimes MN antigens may be associated with a secondary disease, such as Neural-epidermal-growth-factor-like 1 (NELL1)-associated MN linked with a malignancy, and EXT1/ EXT2-, Sema3B-, or even PLA2R associated MN linked with an autoimmune disease. Identifying an association with these antigens may help treat the disease, such as EXT1/EXT2-associated MN with class V lupus nephritis and NELL1-associated MN with malignancy or undetermined antigen associated with hepatitis or no steroidal anti-inflammatory drug use [3]. Here, we will discuss a case that increased the curiosity to determine the antigen responsible for MN leading to proper management.
2. Case Presentation
Mr. YZ, a 65-year-old man, presented to the emergency department with sudden onset of the lower limb and scrotal swelling (1 week) associated with a pruritic rash on both legs. His medical history was significant for diabetes, hypertension, chronic kidney disease stage 2, schizophrenia, benign prostatic hypertrophy, and prior suicidal ideations. His medications were lisinopril. amlodipine, insulin, furosemide. He had been using NSAIDs for several months. He was also using over-the-counter multivitamins. There was no history of malignancy, though his family history was positive for colorectal carcinoma. Examination revealed bilateral pedal edema with an erythematous rash and multiple excoriations from pruritus. The systemic examination was unremarkable. At presentation, lab work revealed blood sugar of 168 mg/dl, HbA1c of 12.3%, serum albumin of creatinine of 2.4 mg/dl, and normal electrolytes (sodium of 138 mEq/L and potassium of 4.0 mEq/ L). His baseline creatinine was known to be about 1.4 mg/dl. Urinalysis showed protein +3 and RBC +1. Workup for glomerulonephritis (ANA, ANCA, HIV, Hep B, C, C3, C4 complement) was negative. The patient had a 24-hour urine protein of 21gm. A skin biopsy was done, showing lipo-dermatosclerosis. Following this, a renal biopsy was done. The report was significant for MN. Immunotyping was negative for PLAR2 and THSD7 negative. Further fixation revealed NELL1 positivity.
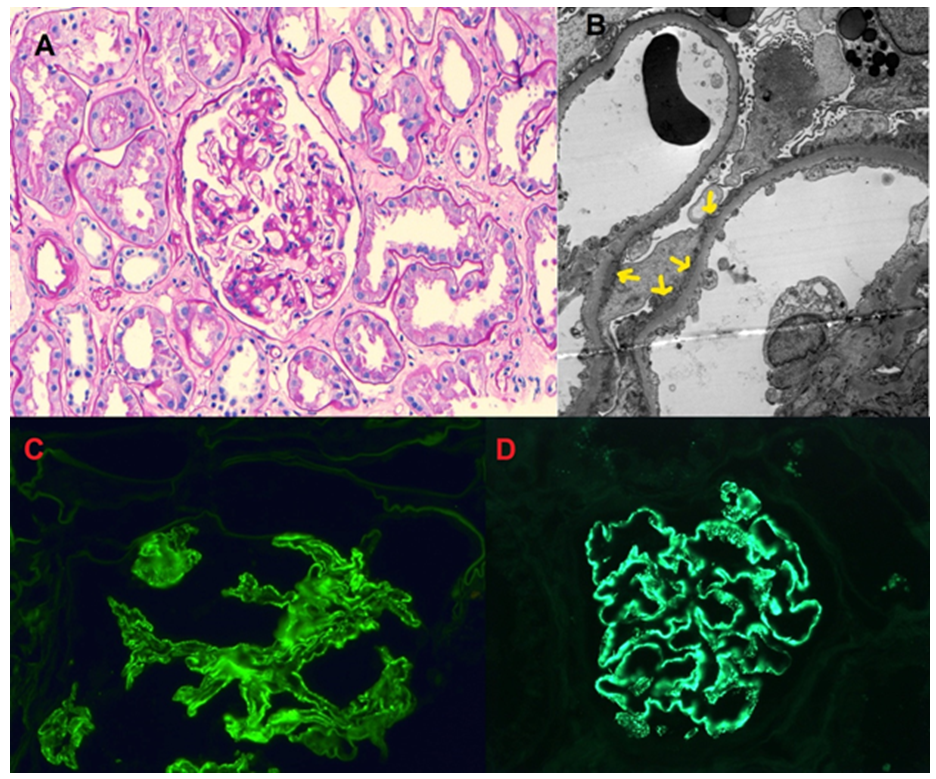
The biopsy results are shown in Figure A-D.
Figure A: Glomerulus with global capillary loop thickening and mild mesangial matrix expansion, PAS stain, 10X.
Figure B: Segmental sub-epithelial electron-dense deposits with extensive foot process effacement.
Figure C: Segmental capillary loop staining, IGG, 400X.
Figure D: Positive NELL1 staining in capillary loops
Extensive workup for malignancy, including CT chest abdomen and colonoscopy, was negative. NSAIDs were stopped along with over-the-counter medication. We found lipoic acid supplements on detailed medication reconciliation. The patient was treated with furosemide, lisinopril, insulin, atorvastatin, diltiazem, and plasma apheresis (PLEX) therapy with additional steroids. We initially considered cyclophosphamide with a steroid taper plan. However, due to poorly controlled hyperglycemia, we had to treat it with rituximab and tacrolimus regimen. Repeat 24hr urine protein before discharge decreased to 11gm and creatinine of 1.7mg/dl.
On follow-up, he improved clinically, and the pedal edema subsided. The proteinuria further decreased to 6.0 gm.
3. Discussion
Nephrotic syndrome is one of the most prevalent disorders with several causes. MN is the most common glomerular disease, which may occur coincidently with neoplasms, NSAIDS, medications, paraproteinemia, and infections [1]. First reported by Lee et al. in 1966, 11% of patients with nephrotic syndrome had an underlying carcinoma. Ro et al. showed that 22% of patients with MN aged 60 years and above had chances of malignancy 10-fold higher than that in age-matched controls [4,5]. Other characteristics of malignancy-associated MN include phospholipase A2 receptor (PLA2R) negativity and IgG1-and IgG2- restricted subclass predominance. [5,6] Because of this close association; our patient underwent an extensive search for malignancy with age-appropriate screening tests. Moreover, we also found PLA2R and THSD7A negative on biopsy staining. A connection between NELL1-MN and male predominance was also observed by Caza et al., especially in cancer patients [2]. Wang et al. found more female patients without malignancy. There are some case reports of medication-related MN as well [10]. Lipoic acid (LA) is considered an antioxidant and insulin-mimetic supplement, works by acting on superoxide radicals, and is proven beneficial in ischemic reperfusion injury and complications related to diabetes [3] Spain et al. report the development of unexpected proteinuria in 3 patients with multiple sclerosis (MS) receiving LA for MS. The critical finding on the kidney biopsies of these patients had a histologic pattern of membranous Nephropathy (MN) and stained positive for neural epidermal growth factor-like 1 (NELL1). For most cases with NELL1 positivity, no significant extra glomerular staining was demonstrated along tubular basement membranes, Bowman's capsule, or vessels. Electron microscopy shows severe podocyte foot process effacement (>50%) in NELL1-associated MN and a higher incidence of mesangial deposits with no sub-endothelial deposits [1]. These findings were found in our case as well (Figure A-D). Regarding the treatment of MN, patients should be treated for hypertension, edema, cardiovascular events, and thromboembolism risks. Angiotensin-converting enzyme inhibitors are recommended for controlling blood pressure and proteinuria [7]. But a decrease in proteinuria may not exceed 30% compared to pre-treatment values, particularly in patients with proteinuria >10 g/24 hr. We started our patient on ACEIs, statins for hypercholesteremia, and apixaban as an anticoagulant [7]. In our case, the patient was also on antioxidants having lipoic acid, and chronic use of NSAIDs [6,7]. In the prior known study, MN reversed when supplements were stopped, and the patients were not started on immunosuppression. A critical treatment component is immunosuppressive therapy, including alkylating Agents (Cyclophosphamide or Chlorambucil), corticosteroids, and other agents. Ponticelli et al. demonstrated the benefit of a 6-month regimen of alternating alkylating agents (cyclo-phosphamide or chlorambucil) with intravenous corticosteroids for achieving remission in MN patients. But it comes at the cost of increased risk of opportunistic infection, reactivation of viral hepatitis, alopecia, gonadal damage, hemorrhagic cystitis, neoplasia, and toxic hepatitis [8] Combining corticosteroids with an alkylating agent has been the most commonly used therapy to preserve kidney function long-term. Over recent years, anti-CD20 biotherapy, particularly with Rituximab, has become popular as first-line therapy because of its safety profile [8].
Rituximab causes the depletion of B cells by antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and apoptosis [8]. One of the studies on using Rituximab in MN evaluated the effects of an infusion (375 mg/m2) every four weeks in 8 patients on primary MN, causing a decrease in urinary protein from 8.6 g/day at baseline to 3.7 g/day at 20 weeks. Complete remission was achieved in 3 patients and partial remission in the other [3]. The KDIGO Guidelines recommend treatment according to the risk score. Treatment depends on patient characteristics, drug availability, and physician preferences in high-risk patients. Immunosuppressive agents are not needed for low-risk. The recommendations include Rituximab on the same level as cyclophosphamide in moderate to high-risk patients. Additionally, the MENTOR trial has confirmed the high relapse rate in patients treated with cyclosporine, thus moving that lower in the treatment arsenal [9]. It is not recommended to be used as monotherapy in higher-risk patients but in combination with Rituximab. In our patient, as we had to elect to use a steroid-sparing regimen due to his severely uncontrolled diabetes, we treated this high-risk case with Rituximab and tacrolimus and found this to be quite effective.
4. Conclusion
MN is one of the most important causes of nephrotic syndrome. It is vital to distinguish between primary and secondary MN to manage the patient properly. It's essential to look for antibodies against antigens like NELL 1 if PLA2R and THSD7A antibodies are negative. Lipoic acid supplements, frequently prescribed in diabetic populations, may cause MN. Close monitoring for proteinuria must be done to detect the disease in its early phases. Moreover, in moderate-risk cases, Rituximab and cyclophosphamide may be equally effective, and Rituximab with tacrolimus may allow a steroid-sparing regimen.
Availability of Data and Materials:
Available in chart.
Competing interests:
None. Authors report no conflict of interests
Funding:
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Dr. Umair Ali wrote the first draft. Dr. N Gokden provided the pathology section images and write-up. Dr. J Arthur and M Singh reviewed and did final edits, including discussion. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Ethics approval and consent participate
Not applicable. Institutional policy does not require ethics approval for anonymized case reports.
Preprint:
This article was uploaded at the below given link as a preprint to generate engagement- it has not been peer reviewed or under consideration in any other journal at this time.
https://www.researchsquare.com/article/rs-2939415/v1
References
- Bomback AS, Fervenza FC. Membranous Nephropathy: treatment approaches. Am J Nephrol 2018; 47(suppl 1):30-42. DOI: 10.1159/000481635
- Caza TN, Hassen SI, Dvanajscak Z, Kuperman M, et al. Nell1 is a target antigen in malignancy-associated membranous Nephropathy. Kidney International (2021) 99, 967-976. https://doi.org/10.1016/j.kint.2020.07.039
- New ''Antigens'' in Membranous Nephropathy Sanjeev Sethi JASN 32: 268-278, 2021. doi: https://doi.org/10.1681/ASN.2020071082
- Lee JC, Yamauchi H, Hopper J Jr. The association of cancer and the nephrotic syndrome. Ann Intern Med. 1966; 64:41-51.
- Lonnbro-Widgren J, Ebefors K, Molne J, et al. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous Nephropathy. Clin Kidney J. 2015; 8:433-439.
- Spain RI, Andeen NK, Gibson PC, Solomon R, et al. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous Nephropathy. Kidney Int. 2021; 100:1208-13. https://doi.org/10.1016/j.kint.2021.10.010
- Rojas-Rivera JE, Carriazo S, Ortiz A. Treatment of idiopathic membranous Nephropathy in adults: KDIGO 2012, cyclophosphamide and cyclosporine A are out, Rituximab is the new normal. Clinical kidney journal. 2019 Oct; 12(5):629-38.
- Scolari F, Delbarba E, Santoro D, Gesualdo L, Pani A, Dallera N, Mani LY, Santostefano M, Feriozzi S, Quaglia M, Boscutti G. Rituximab or cyclophosphamide in the treatment of membranous Nephropathy: the RI-CYCLO randomized trial. Journal of the American Society of Nephrology. 2021 Apr 1;32(4):972-82.
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Jefferson, J.A.; Gipson, P.E.; Rizk, D.V.; Sedor, J.R.; et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 36-46.
- Caza, T. N., & Larsen, C. P. (2022). Lipoic acid in neural epidermal growth factor-like 1-associated membranous Nephropathy: more than a coincidence?. Kidney international, 101(2), 418-419. https://doi.org/10.1016/j.kint.2021.12.001
- Preprint: https://www.researchsquare.com/article/rs-2939415/v1


 Impact Factor: * 2.3
Impact Factor: * 2.3 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 