The Susceptibility of Swine Abortion-Associated Leptospira hyos to Disinfectants
Article Information
Gemerlyn G Garcia1*, Apol Joann Guimpayan1, Lara Joy Baysa1, Lilibeth L Gumpal2
1College of Veterinary Science and Medicine, Central Luzon State University 3120, Science City of Muñoz, Nueva Ecija, Philippines
2Isabela State University, Adanganan Campus 3307, Isabela, Philippines
*Corresponding Author: Gemerlyn G. Garcia, College of Veterinary Science and Medicine, Central Luzon State University 3120, Science City of Muñoz, Nueva Ecija, Philippines
Received: 16 October 2021; Accepted: 01 November 2021; Published: 14 December 2021
Citation: Gemerlyn G Garcia, Apol Joann Guimpayan, Lara Joy Baysa, Lilibeth L Gumpal. The Susceptibility of Swine Abortion-Associated Leptospira hyos to Disinfectants. Archives of Veterinary Science and Medicine 4 (2021): 111-120.
View / Download Pdf Share at FacebookAbstract
Evaluation on the sensitivity of the S-shaped and hook-ended swine abortion-associated Leptospira hyos to disinfectants was undertaken and validated by monitoring recovery counts in McFarland units (MFU). The disinfectants for evaluation included phenol, quaternary ammonium, formaldehyde, chlorine and potassium alum, each prepared in 10, 25 and 50% solutions in sterile deionized water and the pathogen reaction to the disinfectants was evaluated after 30 min and 1 hr exposure time. Results show that in the first 30 min, comparably higher L. hyos MFU was associated with the application of 10 and 25% phenol compared to significantly lower MFUs obtained with 50% phenol. The susceptibility of L. hyos to quaternary ammonium was shown by comparably higher MFUs relative to the application of 10 and 25% quaternary ammonium in contrast to the significantly lower MFU relative to the application of a higher quaternary ammonium concentration (50%). Higher L. hyos MFU was also associated with the application of 10% formaldehyde in contrast to the significantly lower MFU obtained relative to 25% formaldehyde application, with the least MFU linked with the 50% formaldehyde concentration. A trend marked by significantly higher L. hyos MFU was linked to the application of 10% chlorine, a lower MFU associated with 25% chlorine, while the least MFU recorded with 50% chlorine. The application 10 and 20% potassium alum contributed to comparable and significantly higher L. hyos MFU contrary to nil MFU related with 50% potassium alum application. Data obtained after 1 hr application of phenol, quaternary ammonium, formaldehyde, chlorine and potassium alum in 10%, 25% and 50% concentrations contributed to the recorded nil MFUs of L. hyos compared to data obtained in the first 30-minute exposure. These data highlight the significant action of the disinfectants on L. hyos which were apparently activated by disinfectant concentration and duration of exposure.
Keywords
Chlorine; Disinfectants; Formaldehyde; Leptospira hyos; McFarland units; Phenol; Potassium Alum; Quaternary ammonium
Chlorine articles; Disinfectants articles; Formaldehyde articles; Leptospira hyos articles; McFarland units articles; Phenol articles; Potassium Alum articles; Quaternary ammonium articles
Article Details
1. Introduction
Leptospirosis is a globally important zoonotic disease which is reportedly caused by the pathogenic spirochetes of the genus Leptospira [1]. As a disease with sporadic in nature, leptospirosis is reported as an under-diagnosed disease in developing countries like the Philippines. Global warming that leads to extreme weather events such as cyclones, floods, increased rainfall, increased world population and urbanization are considered as the factors associated with upsurge in the incidence of leptospirosis [2]. Leptospirosis outbreaks usually happen during the rainy seasons in tropical regions and in late summer to early fall in temperate regions, but most of the time, outbreaks follow periods of excess rainfall [3]. The pathogenic leptospirae enter their reservoir hosts like rats, infect the renal tubules of these animals and are readily shed in the urine of these hosts. The release of the pathogen in the environment, in moist soil and surface water where human activities related to animal farming, butchering, pest control and aquaculture render people and susceptible animals at risk for leptospira infection. Experts believe that Leptospira infection in pregnant sows is often unnoticed and is only recognized when infections advance which are often accompanied by successive cases of abortion in production areas [4]. Unwittingly, the accessibility of Leptospira rat vectors on detritus from aborted sows in a farm with faulty rodent control may provide a portal for the transmission of leptospirosis between susceptible animals and among animal handlers in the same workplace, under favorable environmental conditions. Leptospira pathogens may continue to have a major impact on the health of food-producing animals and on people who live in areas where control programs for Leptospira may not exist. The barriers to the control of leptospirosis are believed to be precipitated by inadequate diagnostic capacity, lack of concerted efforts to assess the impact of leptospirosis on animal health and limited research that explores control and treatment measures against the pathogen. Identification of disinfectants that potentially eliminate Leptospira is deemed necessary in situations where plans for the prevention or control program against the infection are initiated. Effort to develop a control strategy against leptospira-associated abortion in swine production is wanting. A research-generated information directed to the identification of the most practical method of disinfectant application in areas of infection, in places where transmitters of the infection thrive and in flood-prone areas as control or preventive measure against the Leptospira pathogen is important. Work on this may essentially provide a basis for giving a recommendation for a low-cost strategy that minimizes the transmission of pathogenic Leptospira.
2. Materials and Methods
2.1 Bacterial strains used in the study
Pure cultures of Leptospira hyos (Accession number MK629955) which was utilized in a previous study to evaluate its susceptibility to antibiotics [5] were recovered and used for this experiment.
2.2 Cultivation and microscopic evaluation of Leptospira hyos
The recovered samples were grown in replicated tubes that contained freshly-prepared Fletcher’s medium supplemented with 8% rabbit serum. The samples were kept in an incubator at 31ºC for 24 hours. When the growth of L. hyos in the culture medium was already indicated by the presence a dinger zone within the period of incubation, the samples were collected and washed by centrifugation at 3000 rpm for 15 minutes.The bacterial sediments in tubes were obtained in small aliquots (15 to 20 µL) and placed as smears on slides. The slides were stained using the procedures of others [6] with slight modifications. The smears were fixed in 1% formalin before the application of 5% Sodium Bicarbonate. About 10 drops of basic fuchsin solution was placed on the smear, allowing the stain to adhere on the smear for 5 minutes at RT. The slides were washed with water and allowed to dry before microscopic evaluation.
2.3 In-vitro evaluation on the susceptibility of Leptospira hyos to disinfectants
At the start of this experiment, sample sediments of L. hyos were obtained from replicated tubes before centrifugation at 12,000 x g for 2 minutes. Samples of the sediments were re-suspended in 5 mL sterile deionized water (Thermo Fisher Scientific, USA) for the evaluation of cell density using McFarland standards while most of the samples were obtained as the test organism for the evaluation of the pathogen susceptibility to disinfectants. The inability of L. hyos to form colonies on the surface of a solid medium required the need to lay fresh culture of the pathogen (4.33 + 0.57 MFU) in 1 mL Fletcher’s medium and monitor cell density (expressed in McFarland Units/MFUs) using McFarland standards. Five (5) different kinds of disinfectants obtained from local distributors, each with the 5 described different modes of action were used in the study (Table 1).
|
Disinfectants (Availability/ Distributor) |
Mode of Action |
|
Phenol (3% solution, Lab Alley) |
Inactivates bacterial intracytoplasmic enzyme |
|
Quaternary ammonium (10% solution, Satol) |
Damages the outer membranes of Gram-negative bacteria |
|
Formaldehyde (37% solution, Lab Alley) |
Causes lethal interaction with bacterial protein and nucleic acids |
|
Chlorine (5% solution, Spectrum) |
Oxidizes sulfhydryl enzymes and amino acids |
|
Potassium alum (10% solution, Focus Health Group) |
Reduces the acidity of the bacterial cell wall |
Table 1: Disinfectants used in the evaluation of L. hyos susceptibility to disinfectants.
Samples from each of the five aforementioned disinfectants (Phenol, Quaternary ammonium, Formaldehyde, Chlorine and Potassium alum) were separately obtained and dispensed in sterile tubes as pure preparations in 5-mL volumes at the start of the experiment. From each of these disinfectants, concentrations of 10%, 25% and 50% per mL of sterile deionized water (Thermo Fisher Scientific, USA) were separately prepared as solutions for the test.
For each concentration of disinfectant described, the disinfectant was added in 100 µL-volumes correspondingly to three replicated tubes that contained 1 mL Fletcher’s medium with a fresh culture of L. hyos suspension (4.33 + 0.57 MFU). McFarland readings were taken before disinfectant application and were used as reference in comparing the cell density of L. hyos recovered after exposure to the 5 different disinfectants which were set at different concentrations, for the 30-minutes and 1-hour experiment duration.
2.4 Statistical analysis
McFarland readings relative to the application of the 3 different concentrations of the 5 different disinfectants were expressed as mean McFarland units (MFU) of 3 replicates. Differences in MFUs as an effect of the application of different concentrations of disinfectants were statistically analyzed using LSD (Least significant differences) while differences in MFUs across time intervals (30-minutes and 1-hour) within treatments were analyzed by Students’ T-test (P< 0.01).
3.Results and Discussion
3.1 Results
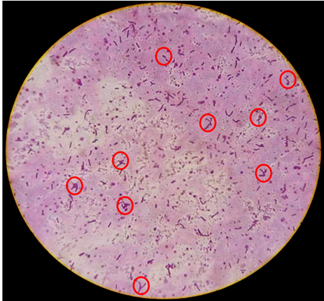
3.1.1 Morphology of the abortion-associated L. hyos: Result of the microscopic evaluation of L. hyos showed that the pathogen appeared S-shaped, short but some were moderately elongated with hooked or curved ends (Figure 1). The organism appeared dark pink to purple after application of the recommended stain.
3.1.2 In-vitro susceptibility of Leptospira hyos to disinfectants: Table 2 provides a summary of the response of the test pathogen L. hyos to the different disinfectants applied at different concentrations, demonstrating data on cell density expressed in McFarland units (MFU). In the first 30 min, the response of L. hyos to phenol was marked by comparably higher L. hyos MFU associated with the application of 10 and 25% phenol compared to significantly lower MFUs obtained with a higher phenol (50%) concentration. The susceptibility of L. hyos to quaternary ammonium was also marked by comparably higher MFUs relative to the application of 10 and 25% quaternary ammonium in contrast to the significantly lower MFU obtained as a result of the application of a higher quaternary ammonium (50%) concentration. Higher L. hyos MFU was also associated with the application of 10% formaldehyde in differentiation to the significantly lower MFU obtained as a result of the application of a higher formaldehyde (25%) concentration, with the least MFU related to the application of the highest formaldehyde (50%) concentration. A trend marked by significantly higher L. hyos MFU was linked to the application of chlorine at a concentration as low as 10% and lower MFU associated with 25% chlorine, with the least MFU recorded with a high chlorine concentration of 50%. The application of potassium alum at 10 and 20% concentrations contributed to comparable and yet significantly higher L. hyos MFU contrary to nil MFU associated with the application of 50% potassium alum. The foregoing MFU data taken after 30-minutes exposure of the test pathogen L. hyos to phenol, quaternary ammonium, formaldehyde, chlorine and potassium alum prepared at the described concentrations, highlight the participation of disinfectant concentration on the disinfectant action of the disinfectants on L. hyos.
|
Disinfectant (Availability/ Packaging) |
Concentration (%, in sterile Deionized water) |
Duration of Interaction |
|
|
30 min |
1 hr |
||
|
Phenol (3% solution)
|
10 |
1.66 (0.12) a,x |
0.00 (0.00) y |
|
25 |
1.66 (0.12) a,x |
0.00 (0.00) y |
|
|
50 |
0.44 (0.09) b,x |
0.00 (0.00) y |
|
|
Quaternary ammonium (10% solution) |
10 |
2.00 (0.16) ab,x |
0.00 (0.00) y |
|
25 |
2.22 (0.09) a,x |
0.00 (0.00) y |
|
|
50 |
1.44 (0.04) b,x |
0.00 (0.00) y |
|
|
Formaldehyde (37% solution)
|
10 |
1.66 (0.02) a,x |
0.00 (0.00) y |
|
25 |
1.33 (0.02) b,x |
0.00 (0.00) y |
|
|
50 |
0.66 (0.02) c,x |
0.00 (0.00) y |
|
|
Chlorine (5% Sodium hypochlorite)
|
10 |
1.22 (0.02) a,x |
0.00 (0.00) y |
|
25 |
0.77 (0.12) b,x |
0.00 (0.00) y |
|
|
50 |
0.33 (0.08) c,x |
0.00 (0.00) y |
|
|
Potassium alum (10% Potassium Alum) |
10 |
1.33 (0.03) a,x |
0.00 (0.00) y |
|
25 |
1.11 (0.24) ab,x |
0.00 (0.00) y |
|
|
50 |
0.00 (0.00) b,x |
0.00 (0.00) y |
|
|
Fletcher’s medium (Reference control) |
|
4.33 (0.57) x
|
4.24 (0.53) x
|
Table 2: Cell density (MFUs) of L. hyos in response to its exposure to the different concentrations of disinfectants at indicated durations of interaction. Values represent the mean (± standard deviation) cell density of Leptospira hyos expressed as McFarland units (MFU) recovered after the application of different disinfectants given at different concentrations at specified duration of interaction. MFU of 0.5 is equivalent to 1.5 x 108 CFU/ml; 1.0 MFU/3.0 x 108 CFU/ml; 2.0 MFU/6.0 x 108 CFU/ml; 3.0 MFU/9.0 x 108 CFU/ml; 4.0 MFU/12.0 x 108 CFU/ml; 5.0 MFU/15 x 108 CFU/ml; 6.0 MFU/18.0 x 108 CFU/ml; 7.0 MFU/21.0 x 108 CFU/ml; 8.0 MFU/24.0 x 108 CFU/ml; 9.0 MFU/27.0 x 108 CFU/ml; and 10.0 MFU/30.0 x 108 CFU/ml. a, b, c (significant differences in cell density as an effect of the concentrations of each disinfectant in a given time point, P<0.01). x, y (significant differences in cell density as an effect of the duration of interaction between pathogen and the disinfectants (30 min and 1 hour, P<0.01).
The time in monitoring the effect of the same disinfectants was extended for another 30 minutes to complete a 1-hour data. Data in Table 2 demonstrate nil L. hyos MFUs recorded and related with the appli-
cation of phenol, quaternary ammonium, formaldehyde, chlorine and potassium alum prepared at 10%, 25% and 50% concentrations. These data demonstrate significantly reduced mean L. hyos MFUs as a result of the application of phenol, quaternary ammonium, formaldehyde, chlorine and potassium alum, prepared at 10%, 25% and 50% concentrations, compared to data obtained after the first 30-minute exposure. These data also demonstrate the contribution of duration of exposure on the disinfectant action against L. hyos.
4. Discussion
The study explored the susceptibility of L. hyos to disinfectants as an adjunct procedure after determining the responses of the same swine abortion-associated pathogen to antibiotics [5]. Research-generated information are extremely needed in order to draw a basis in giving treatment in the form of antibiotics or as a disinfection control strategy in animal production units.
The study involved evaluation of the responses of L. hyos to a set of disinfectants that included phenol which inactivates bacterial intracytoplasmic enzymes; quaternary ammonium that impairs outer membranes of Gram-negative bacteria; formaldehyde that reportedly causes lethal interaction with bacterial protein and nucleic acids; chlorine that oxidizes bacterial sulfhydryl enzymes and amino acids; and potassium alum that reduces the acidity of bacterial cell walls, all prepared at 10%, 25% and 50% concentrations, where bacterial cell densities were monitored post-exposure to the disinfectants as a measure to determine disinfectant action on the test pathogen at specified periods of observation.
It has been established that both disinfectant concentration and duration of exposure determine the susceptibility of L. hyos to disinfectant action. It was found out that high disinfectant concentrations were generally related to low cell densities of recovered L. hyos which were expressed as MFUs, correspondingly pointing to a greater microbial death as a result of disinfectant action. Some differences in disinfectant action were, however, noted. Some disinfectants like phenol, quaternary ammonium and potassium alum have lower concentrations (10 and 25%) that can comparably exert a disinfectant action on L. hyos. Other disinfectants like formaldehyde and chlorine exert significantly different disinfectant action on L. hyos relative to the application of the 3 different concentrations of disinfectants (10, 25 and 50%). Results of the study provided a way in defining a 10% disinfectant concentration as the MIC (minimum inhibitory concentration) for all the 5 disinfectants tested against L. hyos. It was also found out that the 5 disinfectants exert their optimum disinfectant action on L. hyos within the first 30-minutes exposure time.
Efforts intended to identify effective disinfectants against Leptospira spp. in different work settings have been undertaken by many researchers. One of these studies includes a study [7] which reportedly inactivated Leptospira spp. with formalin and phenol combination although concentrations of the disinfectants were not discussed. The effectiveness of disinfectants was reportedly tested [8] in a study where urine of dogs with leptospirosis were inactivated with by 10% bleach solution. The effect of chlorine against Leptospira spp. in soil and water habitats was reportedly tested by a group of researchers [9] who described the effective elimination of Leptospira spp. by 2% chlorine solution. Researchers at the Center for Food Security and Public Health [10] reported that Leptospira spp. in moist heat can be inactivated by disinfectants similarly used in this study like 1% sodium hypochlorite, quaternary ammonium disinfectants and formaldehyde in addition to other disinfectants like 70% ethanol, iodine-based disinfectants, hydrogen peroxide and glutaraldehyde applied for 15 minutes, which were quite different from the duration of exposure undertaken in the present study.
The effect of disinfectants on leptospires was also tested in another study [11] which reportedly used 2 mL solution of calcium hypochlorite containing active chlorine in a concentration that ranged from 2 to 250 mg/L against one drop of a 4 to 6-day culture of Leptospira icterohaemorrhagiae or L. grippotyphosa in Korthof medium which were later determined by sub-inoculation and microscopic examination. In a similar experiment [12], 0.1% sodium hydroxide solution reportedly killed all the Leptospira spp. in 20 seconds.
There are no recent studies that described the susceptibility of Leptospira spp. to potassium alum. However, there are studies that describe the effectiveness of potassium alum in inactivating some Gram-negative and Gram-positive bacteria. In another study [13], it was reported that white alum was potent against Escherichia coli O157:H7 at a concentration above 1% which described the effect in a dose- and time-dependent manner. The work of other researchers [14] reported the effectiveness of bis-quaternary ammonium on hospital isolates of Pseudomonas aeruginosa based on bactericidal effects and toxicity in human epithelial cells using lactate dehydrogenase release (LDH). The study of other researchers [15] described the use of phenol compounds as anti-bacterials which exert antimicrobial activities that denature and coagulate proteins in a wide range of Gram-positive and Gram-negative bacteria.
The application of potassium alum against Leptospira hyos in this study is a new finding as the potential of potassium alum as a disinfectant is not widely explored. This is a chemical that can be acquired over the counter which can be applied as a disinfectant in farm premises at 10% MIC-defined concentration to minimize L. hyos infections that can be acquired through contamination of farm premises.
5. Conclusion
A report that discussed the susceptibility of the swine abortion-associated L. hyos to disinfectants with different mechanisms of action was made. The significant reduction of cell densities relative to the application of disinfectants at described concen-trations and at different duration of exposure, and the identification of the MICs of the disinfectants provide a basis in giving a recommendation for a control program against L. hyos in swine farms.
Acknowledgement
This work was undertaken with the assistance of the Veterinary Microbiology Section, College of Veterinary Science and Medicine, Central Luzon State University, Philippines.
Conflict of Interest
No conflict of interest exists among the authors relative to the submission of this paper for publication.
References
- Adler B, de la Pena MA. Leptospira and Leptospirosis. Veterinary Microbiology 140 (2010): 287-296.
- Lau CL, Smythe LD, Craig SB, et al. Climate change, flooding, urbanization and leptospirosis: fueling the fire? Transactions of the Royal Society of Tropical Medicine and Hygiene 104 (2010): 631-638.
- Mason MR, Encina C, Sreevatsan S, et al. Distribution and diversity of pathogenic Leptospira species in peridomestic surface waters from South Central Chile. PLoS Neglected Tropical Diseases 10 (2016): 8.
- Levett PN, Branch SL, Whittington CU, et al. Two methods for rapid serological diagnosis of acute leptospirosis. Clinical Diagnostic Laboratory Immunology 8 (2001): 349-351.
- Garcia GG, Dioses MI. The involvement of Leptospira spp. in swine abortion and susceptibility of the pathogen to antibiotics. Archives in Veterinary Science and Medicine 4, 3 (2021): 71-84.
- Ryu E. A simple method for staining Leptospira and Treponema. Japanese Journal of Microbiology 7, 2 (1963): 81-85.
- Baskerville A. Histopathological aspects of diagnosis of leptospirosis. Current Topics in Veterinary Medicine and Animal Science 36 (1986): 33-43.
- Guerra M. Leptospirosis. Journal of American Veterinary Medical Association 234 (2009): 472-477.
- Yuwvaranni S, Thiruvengadam S. Distribution, isolation, identification, characterization and effect of Chlorine, Antibiotics and herbs on bacterial genus Leptospira in Chennai. International Journal of Chemical Sciences 8, 5 (2010): S520-S526.
- Researchers at the Center for Food Security and Public Health (2013).
- Kmety E, Keleti J. The effect of disinfectants on leptospires. Ceskoslovenska Epidemiologie Mikrobiologie 5, 6 (1978): 295-300.
- Heuer C, Wilson PR, Bensc hop J, et al. Leptospirosis - a rising public health hazard? Proceedings of the Food Safety, Animal Welfare and Biosecurity Branch. New Zealand Veterinary Association 273 (2008):101-106.
- Shahriari R, Salari S, Shahriari S. In vitro study of concentration-effect and time-course pattern of white alum on Escherichia coli 0157:H7 growth. African Journal of Traditional Complementary and Alternative Medicine 14, 2 (2017): 311-318.
- Murakami K, Yumoto H, Murakami A, et al. Evaluation of the effectiveness of the potent bis-quaternary ammonium compound, 4,4'-(α,ω-hexamethyl-ene-dithio) bis (1-octylpyridinium bromide) (4DTBP-6,8) on Pseudomonas aeruginosa. Journal of Applied Microbiology 122, 4 (2017): 893-899.
- Sabbineni J. Phenol an effective antibacterial agent. Research and Review. Journal of Medicinal and Organic. Chemistry 3, 2 (2016): 182-191.

 Impact Factor: * 1.1
Impact Factor: * 1.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 