SARS COV-2 and Inflammation: Its Impact on the Cardiovascular System
Article Information
Muneeb Qadir1, Saumya Bhagat1, Huma Quasimi1, Intzar Ali2, Afreen Khan3, Mairaj Ahmed Ansari4, Iqbal Alam1*
1Department of Physiology, Hamdard Institute of Medical Sciences and Research, New Delhi, India
2Department of Microbiology, Hamdard Institute of Medical Sciences and Research, New Delhi, India
3Department of Medicine, Hamdard Institute of Medical Sciences and Research, New Delhi, India
4Department of Biotechnology, Hamdard University, New Delhi, India
*Corresponding author: Md Iqbal Alam, Ph.D., Professor and Head, Department of Physiology, Hamdard Institute of Medical Sciences and Research, New Delhi-110 062, India
Received: 22 March 2022; Accepted: 30 March 2022; Published: 14 April 2022
Citation: Muneeb Qadir, Saumya Bhagat, Huma Quasimi, Intzar Ali, Afreen Khan, Mairaj Ahmed Ansari, Iqbal Alam. SARS COV-2 and Inflammation: Its Impact on the Cardiovascular System. Cardiology and Cardiovascular Medicine 6 (2022): 157-170.
View / Download Pdf Share at FacebookAbstract
The novel corona virus is identified as a positive–sense single–stranded RNA virus and member of the coronavirus family. The virus is thought to have originated from Wuhan, China, and acquired the ability of human–to–human transmission. Although most patients with SARS–CoV–2 (previously known as “2019 novel coronavirus”) manifest fever and respiratory tract symptoms. SARS–CoV–2 infection may also involve other organs/systems and present with extra–respiratory manifestations including cardiac, gastrointestinal, hepatic, renal, neurological, olfactory, gustatory, ocular, cutaneous and hematological symptoms. The severe risk factors are commonly detected in elder patients and with medical comorbidities like cancer, hypertension and diabetes. Since the outbreak and rapid spread of SARS–CoV–2, it has been evident that disease prognosis has largely been influenced by multi–organ involvement. Comorbidities such as cardiovascular diseases have been the most common risk factors for severity and mortality. The involvement of different organs of the body is explained based on the presence of ACE–2 (angiotensin–converting enzyme 2) in different tissues and cells. Several extra–respiratory manifestations, such as cardiac involvement, acute kidney injury, coagulation disorders, and thrombotic complications, could be associated with a poor prognosis. This review provides a comprehensive presentation of the pathophysiological effects of SARS–CoV–2 infection on different organs of the body such as CVS (cardiovascular system), CNS (central nervous system), GIT (gastrointestinal tract), Skin, Renal, and Blood.
Keywords
SARS-CoV-2; Hypertension; ACE-2; Multi-organ; Cardiovascular system; cytokine storm
SARS-CoV-2 articles; Hypertension articles; ACE-2 articles; Multi-organ articles; Cardiovascular system articles; cytokine storm articles
Article Details
1. Introduction
Coronaviruses are a family of RNA viruses with a single–stranded positive–sense genome, named for their crown–like appearance. The coronaviruses are known to cause respiratory infections in humans. The novel coronavirus disease 2019 (COVID–19) caused by SARS–CoV–2 resulted in panic and global health concern since December 2019. The SARS–CoV–2 is a zoonotic virus that belongs to the Coronaviridae family that infects humans and several animal species [1].
In December 2019, a group of pneumonia cases caused by SARS–CoV–2, emerged from Wuhan, China. WHO and Coronavirus Study Group officially named the disease as coronavirus disease 2019 (COVID– 19) and SARS–CoV–2, both issued on 11 February 2020. The Chinese scientists isolated SARS–CoV–2 and came out with genome sequencing [2]. It has been well–known that SARS–CoV–2 shares sequence homology with the SARS–CoV and a bat coronavirus [3]. The two highly pathogenic coronaviruses of zoonotic origin such as SARS–CoV and MERS–CoV (Middle East Respiratory Syndrome Coronavirus) were identified earlier which causes extensive epidemics and fatality in many countries. SARS–CoV–2 is the third known highly pathogenic human coronavirus infection, but it differs from previous corona viruses in fatality and transmission rate [4].
SARS outbreak in 2002 was an epidemic and is believed to have a bat as an original reservoir. Later, it acquired mutations and was capable of infecting humans [5]. Almost after a decade, another similar eruption took place named as Middle East Respiratory Syndrome (MERS). MERS is also called the camel flu because its intermediate host was camel [6]. The mortality rate of MERS was around 35 percent. The SARS–CoV–2 is relatively benign with a mortality rate of 1–5 percent as compared to SARS and MERS [7].
2. Pathophysiology
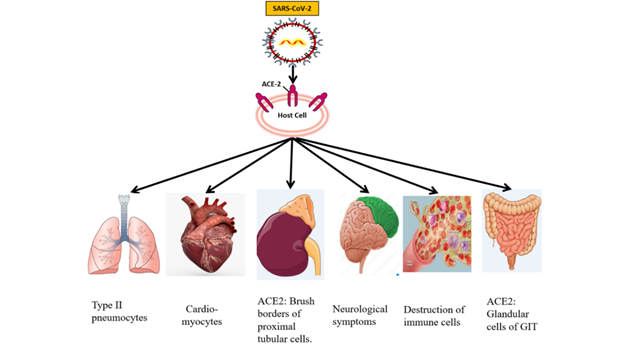
The SARS–CoV–2 has become the global threat infecting millions of people. The lungs are the prime target of SARS–CoV–2 infection. The virus enters into the host cells through the ACE–2 receptor. The ACE2 receptors are abundantly expressed on type II alveolar cells of the lungs. The respiratory symptoms such as cough, sputum, and shortness of breathing remain dominantly present following the fever. However, the extra–respiratory manifestation of SARS–CoV–2 infection also has been observed in most SARS–CoV–2 cases. This indicates the involvement of different organs of the human body. SARS–CoV–2 is primarily a respiratory disease, but a large number of patients showed the symptoms of CVDs including hypertension, acute cardiac injury, and myocarditis. It seems that CVS (heart) is vulnerable to the SARS–CoV–2 infection. Cardiovascular tissues or the cells express the ACE2 receptor and are potentially at risk for SARS–CoV–2 infection. SARS–CoV–2 infection can cause acute cardiac injury, chest pain, and arrhythmic complications. The cardiac involvement in patients with SARS–CoV–2 may be associated with poor prognosis. The SARS–CoV–2 also invades the central nervous system and inducing neurological damage. The SARS–CoV–2 mRNA was detected in brain cells despite the low levels of ACE2. The SARS–CoV–2 also affects the gastrointestinal organs because ACE2 is abundantly expressed on glandular cells of stomach, duodenal and rectal epithelia, as well as on endothelial cells and enterocytes of small intestines. The gastrointestinal symptoms including diarrhea, nausea, vomiting, and abdominal discomfort vary significantly. The clinicians should remain cautious during the management of SARS–CoV–2 patients with GI manifestations, as the viral shedding may occur for a long time. The cutaneous manifestation associated with the SARS–CoV–2 is also reported. However, the dysregulation of the immune system and the production of cytokines lead to skin insults. Cytokines reach the skin and stimulate the dermal cells resulting in erythema, urticaria lesion, vesicles, and others. The recent findings indicate an increased risk of AKI (acute kidney injury) during SARS–CoV–2 infection. Some people who are suffering from severe cases of SARS–CoV–2 showed kidney damage even if the person is not having any underlying kidney diseases. SARS–CoV–2 patients were prone to cause hematological changes. Hematological abnormalities in SARS–CoV–2 patients are lymphopenia, neutrophilia, and thrombocytopenia. Coagulation disorders and thrombotic complications may be associated with poor outcomes in patients with SARS–CoV–2 [7, 8].
3. COVID-19 and Cardiovascular System
SARS–CoV–2 not only causes viral pneumonia but have major repercussion on the cardiovascular system. Patients with CV morbidity including advanced age, diabetes, hypertension, obesity, and cerebrovascular disease have been identified as among the vulnerable populations with increased morbidity and mortality. A wide range of arrhythmias have been reported to complicate the course of SARS–CoV–2. COVID–19 associated cardiac complications have been reported frequently and the mechanisms appear complicated. It includes direct viral injury, hypoxemia, and unstable hemodynamic status with hypo–perfusion, enhanced systematic inflammation, increased endogenous catecholamine, and drug toxicity [9, 10].
The coronaviruses outbreak was associated with a considerable burden of CV co-morbidities and complications [11]. Common cardiac complications in SARS–CoV were hypertension, myocarditis, arrhythmias, and sudden cardiac death. Autopsies of SARS–CoV–2 patients showed infiltration of the myocardium by interstitial mononuclear inflammatory cells.
The myocardial injury and the increased levels of cardiac biomarkers are likely to be associated with SARS–CoV–2 infections [12].
In a study by Shi S, et al. (2020) of 416 patients of whom 57 died, the cardiac injury was a common finding (19.7%) [13]. Zhou F, et al. (2020) reported that 44 (23.0%) of 191 hospitalized patients had heart failure [14]. The patients with COIVD–19 and cardiac injuries are more frequently require mechanical ventilation and susceptible to a higher mortality rate than those without cardiac injuries. ACE2 plays a vital role in the cardiovascular function, immune systems, and the development of hypertension, and diabetes mellitus.
SARS–CoV–2 mainly invades alveolar epithelial cells, resulting in respiratory symptoms. These symptoms are more severe in patients with CVDs, which might be linked with an elevated level of ACE2 in these patients compared with healthy individuals [15].
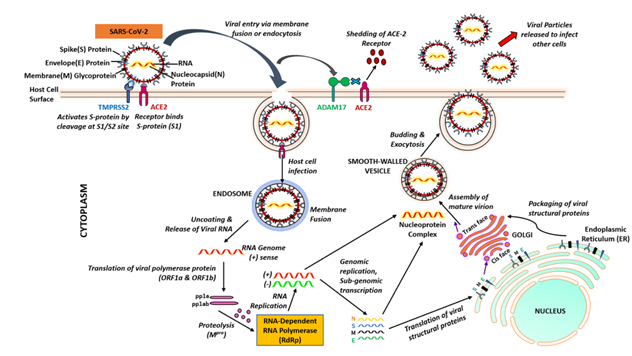
Figure 2: Cellular entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Initially, the SARS-CoV-2 spike protein binds to angiotensin converting enzyme 2 (ACE2) and TMPRSS2 is involved in priming of the S protein, which involves cleavage at the S1/S2 domains. The virus fuses into the cell membrane. Once SARS-CoV-2 enters into cell, it uses the endogenous cellular machinery to replicate itself.
4. Acute Cardiac Injury and Myocarditis in COVID–19
It has been reported that myocarditis/myocardial damage appears after several days of SARS–CoV–2 infection. The mechanisms of SARS–CoV–2–induced myocardial injury may be related to the up regulation of ACE2 in the heart and coronary vessels. Myocardial injury is related to the SARS–CoV–2 occurred in 5 of the first 41 patients diagnosed with SARS–CoV–2 in Wuhan. In this study, four of five patients with myocardial injury were admitted to the ICU (intensive–care unit), which indicates the serious nature of the myocardial injury in patients with SARS–CoV–2 [16]. SARS–CoV–2 induced respiratory failure and hypoxia may also cause damage to the myocardium and immune system. The injury to the heart may be due to ACE2– related signaling pathways.
The other mechanisms of myocardial injury include cytokine storm triggered by an imbalanced response by type 1 and type 2 T helper cells. The respiratory dysfunction and hypoxemia caused by SARS–CoV–2 also result in damage to myocardial cells [17].
5. Chronic Cardiovascular Damage in COVID–19
SARS–CoV–2 infection complications may also be linked with elevated long–term CV risk. The SARS–2002 outbreak demonstrated that patients with a history of SARS–CoV infection often had hyperlipidemia, CV system abnormalities, or glucose metabolism disorders but no long term effects of a SARS–CoV–2 are known yet. A 12 year follow–up survey of 25 patients who recovered from SARS–CoV infection found that 68% had hyperlipidemia, 44% had cardiovascular system abnormalities and 60% had glucose metabolism disorders. It is thought that SARS–CoV–2 might also cause chronic damage to the cardiovascular system [18].
6. Patients with Pre–Existing CVDs
The patients with underlying CVDs can aggravate pneumonia and increase the severity of COVID–19 symptoms. The prevalence of pre–existing hypertension appears to be higher in SARS–CoV–2 patients versus those who do not have CVD [19]. The large number of deaths from SARS–CoV–2 occurs in patients with pre–existing CVDs.
Patients with the acute coronary syndrome suffering from SARS–CoV–2 have a poor prognosis and likely to have cardiac insufficiency, leading to a sudden deterioration and might act as a precipitating factor to worsen the condition and lead to death. Currently, there are many vaccines available throughout world. The anti–viral drug treatment should be closely monitored; many antiviral drugs can cause cardiac insufficiency, arrhythmia, or other cardiovascular disorders [20].
7. Immune System Dysregulation and CVDs in COVID–19
COVID–19 patients are associated with a pro–inflammatory cytokine storm. The activation of immune responses triggers a large series of CVDs including atherosclerosis, heart failure (HF) and hypertension. In severe cases of COVID–19, there is systemic elevation of numerous cytokines such as IL–6, IL–2, IL–7, granulocyte colony–stimulating factor, C–X–C motif chemokine 10 (CXCL10), chemokine (C–C motif) ligand 2, and tumor necrosis factor–α, which result in cytokine release syndrome. Altered vascular permeability can result in non–cardiogenic pulmonary edema and promotes ARDS, ultimately multi–organ dysfunction. Elevated IL–6 in serum is general feature of cytokine release syndrome. IL–6 is a clinical predictor of mortality in SARS–CoV–2, and targeting it may be permissive to tackle the cytokine release syndrome. The role of immune dysregulation in hypertension can provide a possible mechanistic link between immune dysregulation and a more severe course of COVID–19. The hypertension is associated with dysregulation of CD4þ and CD8þ cells which are prone to overproduction of cytokines in SARS–CoV–2 related cases. These immune mechanisms also contribute significantly to accelerated end–organ damage. The large–scale observational studies investigating the association between hypertension and COVID–19 infections and its outcome are to be needed [21].
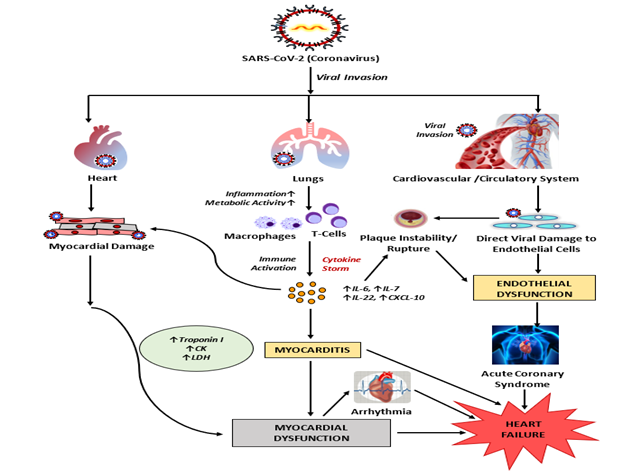
Figure 3: Possible mechanisms of SARS-CoV-2 induced inflammation (in Cardio-myocytes) and cardiovascular damage. SARS-CoV-2 infection of endothelial cells may cause endothelial vascular dysfunction. As SARS CoV-2 enters into the respiratory tract, it leads to systemic inflammation and immune cell over-activation causing cytokine storm resulting in increase in cytokines like IL6, IL7, IL22 and CXCL10. The activated T-cells and elevated cytokines may infiltrate into myocardium resulting in development of myocarditis and cardiac damage.
8. ACE-2 Induced Hypertension and COVID–19
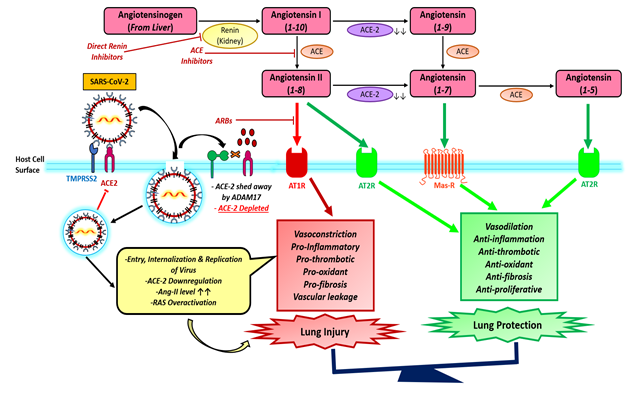
The relationships between hypertension and COVID–19 are unknown but renin–angiotensin–aldosterone system plays an important role in the pathophysiology of hypertension [22]. The RAS mechanism is a much more complex system that control not only blood pressure, homeostasis and cardiovascular function but also plays an important role in the injury of several organs including the lungs. However, Ang 1–7 may contribute to protection from Ang II effects by inducing vasodilatation. Ang 1–7 synthesis release nitric oxide, and activation of baroreflex sensitivity that protect against tissue injury in the cardiovascular system, kidney, and other organs. The entry of SARS–CoV–2 through the ACE2 receptor establishes the link between COVID–19 and the RAS. Kuba K, et al.2005, demonstrated the worsening of lung failure could be attenuated by treatment with an Angiotensen receptor blocker (ARB), clearly demonstrating that the activation of the pulmonary Ang II–AT1R axis influences the pathogenicity of SARS–CoV 2 infection [23].
9. Hematological Effects of COVID–19
The COVID–19 patients areprone to cause hematological changes. The viral infections cause various complications including the effect on different blood cells. The SARS–CoV–2 infection primarily attacks the lymphocytes and platelets. Apart from these common symptoms including fever, fatigue, and dry cough, and dyspnea, and some SARS–CoV–2 patients develop hematological changes including reduced lymphocyte count and platelet count [24].
9.1 Lymphopenia
The first case of lymphopenia caused due to SARS–CoV–2 infection confirmed in the USA developed a slight decrease in white cell count within one week from the onset of diseases. Early research of 41 COVID–19 patients, hospitalized at Jin Yin–tan Hospital indicated leukopenia (accounted for 25%) and lymphopenia (accounted for 63%). The level of lymphocytes (major antiviral cells) continually and severely decreases in ICU and dead patients measured at Zhongnan Hospital of Wuhan University [25].
9.2 Neutrophilia
Various SARS–CoV–2 reports indicate that neutrophilia is likely to appear in ICU cases [26]. A study of 69 confirmed cases of SARS–CoV–2 infection from Singapore showed that ICU patients tend to develop neutrophilia during hospitalization [27].
9.3 Thrombocytopenia
A study that includes 41 SARS–CoV–2 cases reported in (Wuhan Jinyintan Hospital), thrombocytopenia (platelet count < 100 × 109/L) appeared in 5% (2/40) of them [28]. A meta–analysis of 24 studies comprising 2507 patients showed that the decreased platelet counts were observed in 32.3% and 16.4% of critically and non–critically ill SARS–CoV–2 patients, respectively [29]. In most cases, the platelet count remains low but not to the level at which bleeding occurs. Coronaviruses can infect bone marrow cells, resulting in abnormal hematopoiesis.
The mechanisms regarding the involvement of the hematopoietic system in coronavirus remain unclear. There are three hypothesize which are responsible for thrombocytopenia:
(i) Direct infection of bone marrow cells by the virus and inhibition of platelet synthesis
(ii) Platelet destruction by the immune system
(iii) Platelet aggregation in the lungs, resulting in microthrombi and platelet consumption
It is contemplated that hematopoiesis in bone marrow is inhibited by certain receptors to cause decreased primary platelet formation and lead to thrombocytopenia in SARS–CoV–2 as inhibited in previous outbreaks of CoVs. Zhou Y et al analyzed 33 blood samples of severe and critically ill SARS–CoV–2 patients; found that T cells were over–activated after infection, to produce granulocyte–macrophage colony–stimulating factor and interleukin–6 (IL–6) [30]. It is speculated that after the cytokine storm destroying hematopoietic progenitor cells in the bone marrow which could lead to the decrease of peripheral blood platelet count. The damaged pulmonary capillary beds cause the rupture of megakaryocyte and platelet release to be blocked [31]. SARS–CoV–2 infection may increase platelet destruction by increasing the levels of auto-antibodies and immune complexes. The elevated inflammatory response due to viral infection results in lung damage. The damaged pulmonary tissues and cells may activate platelets in the lungs, resulting in aggregation and formation of microthrombi, which increases platelet consumption. The majority of COVID–19 patients have thrombocytopenia and impaired coagulation time [32].
10. COVID 19 and Coagulation
The viral infections cause various complications in critically ill patients which led to the activation of multiple systemic coagulation and inflammatory responses. The SARS COV-2 leads to disseminated intravascular clotting in most of the critically ill patients. The thrombotic complications have been observed in SARS–CoV–2 patients, especially in those who are critically ill [33]. The incidence of thrombotic complications among 148 patients with SARS–CoV–2 in an ICU was 31%, including a venous thrombo–embolism incidence of 27% and an arterial thrombotic event incidence of 3.7% [34].
11. Coagulation and Inflammation
Different viral infection initiates complex systemic inflammatory responses as part of innate immunity. The data from Tang N, et al. (2020), suggest that disseminated intravascular clot occurs in patients in later stages of SARS–CoV–2 infection [35]. The SARS–CoV–2 patients showed significant inflammation, based on elevated levels of IL–6, increased C–reactive protein, erythrocyte sedimentation rate, and elevated fibrinogen. In the early phase of SARS–CoV–2 infection, coagulation test abnormalities are seen and are likely due to profound inflammatory response but clinically bleeding is not observed [36].
The hypercoagulable state in SARS–CoV–2 is still unknown, several mechanisms are proposed. Disseminated Intravascular Clot (DIC), properties of virus itself, activation of complement cascade and endothelial dysfunction may be possible pathophysiology. As virus enters into the cell, it induces the production of fibroblast growth factors and phospholipase A2 (PLA2) resulting in fibrinogen production. The SARS–CoV–2 also stimulates the pro–inflammatory factors like TNF–α, IL–6, IL–1, IL12 resulting in platelet activation. SARS–CoV–2 also enters into platelet and releases thromboxane–A2 from prostaglandins leading the platelet aggregation and vasoconstriction. The cytokine storm, thromboxanes, inflammatory mediators led to the activation of coagulation cascade, result in fibrin production. Fibrin will accumulate and bind platelets, leading to the hypercoagulability and clot formation. Sometimes the clots may travel to the lungs causing hypoxemia and contributing in ventilation/perfusion mismatch and result in ARDS. SARS–CoV–2 virus elicits the systemic inflammatory response and cause an imbalance between procoagulant and anticoagulant homeostatic mechanisms. Various mechanisms are involved, including endothelial dysfunction, von Willebrand factor elevation, Toll–like receptors activation, and tissue–factor pathway activation [37]. Toll–like receptors are expressed in innate immune cells and activation of Toll–like receptors can be induced by large amount of pathogen–associated–molecular patterns present in viruses and other foreign organisms. Toll–like receptors play an important role in initiation of innate immune response with production of inflammatory cytokines by recognizing ssRNA of SARS–CoV–2, therefore likely to be involved in viral clearance [38].
The SARS-CoV-2 enters inside the cell, replicates rapidly and infecting more cells. The host cells to undergo pyroptosis and release damage-associated molecular patterns (DAMPs) due to elevated number of SARS-CoV-2. DAMPs are recognized by pattern recognition receptors (RIG-1, MDA-5, and TLR3) present on lung epithelial cells, triggering the generation of pro-inflammatory cytokines, type I interferon. Many infected patients are asymptomatic mostly young individuals, due to secretion of type 1 interferon and virus replication is inhibited. However, in elderly individuals with comorbid conditions, due to immune suppression, IFN-I production is delayed. It may lead to further recruitment of inflammatory cells which are responsible for the secretion of huge pro-inflammatory cytokines. This overwhelming immune response in SARS–CoV–2 has been linked to what is termed as a cytokine storm. Cytokine storm results in massive and irreversible damage to the multi-organs system. If the multi-organ damage is not treated, may lead multi-organ failure followed by death [39-40]. This exaggerated immune response mediated by SARS-CoV-2 is thought to be increase the severity of diseases and death in patients with SARS–CoV–2 due to elevated levels of circulating cytokines [41].
12. Conclusion
The outbreak of SARS–CoV–2 is an ongoing global public health crisis. There are numerous indications that SARS–CoV–2 also attacks other organs of the body on large scale including heart, blood vessels, CNS, kidney, liver, and skin. Multi–organ involvement is obvious since the outbreak of SARS–CoV–2 and causing extra–pulmonary organ injuries. The progression of SARS–CoV–2 is widely influenced by the presence of co–morbidities. Acute Respiratory Distress Syndrome, heart failure, AKI, shock, coagulation abnormalities and multi–organ failure resulting in death. The clinician should be cautious about the co–morbidities and potential organ damage. Hence, there is an urgent need for the implementation of preventative and protective measures.
Acknowledgements
We would like to thank Dr. G N Qazi, Director General / CEO, HIMSR, Jamia Hamdard, New Delhi for his valuable inputs and support.
Additional Information
The authors declare no competing interests whatsoever.
References
- Vellingiri B, Jayaramayya K, Iyer M, Narayanasamy A, Govindasamy V, et al. COVID-19: A promising cure for the global panic. Science of The Total Environment (2020): 138277.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research 7 (2020): 1-11.
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, et al. The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5 (2019): 536-544.
- Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 38 (2020): 10-18.
- van Staden C. COVID-19 and the crisis of national development. Nature Human Behaviour (2020): 1-2.
- Fehr AR, Channappanavar R, Perlman S. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Annual review of medicine (2017): 68.
- Faiq M, Kumar A, Singh H, Pareek V, Qadri R, et al. COVID-19: A review on molecular basis, pathogenic mechanisms, therapeutic aspects and future projections (2020).
- Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multi-organ response. Current Problems in Cardiology (2020): 100618.
- Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. Journal of Cardiovascular Electrophysiology 5 (2020): 1003-1008.
- Lai CC, Ko WC, Lee PI, Jean SS, Hsueh PR. Extra-respiratory manifestations of COVID-19. International Journal of Antimicrobial Agents (2020): 106024.
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. Journal of the American College of Cardiology 75 (2020): 2352-2371.
- Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA cardiology (2020).
- Shi S, Qin M, Shen B, Cai Y, Liu T, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA cardiology (2020).
- YLiu ZF, ZXiang, JWang, YSong, BGu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (2020): 1054-1062.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiology 17 (2020): 259-260.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 395 (2020): 497-506.
- Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology 136 (2004): 95-103.
- Wu Q, Zhou L, Sun X, Yan Z, Hu C, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Scientific reports 7 (2017): 9110.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet (2020).
- Chan JF, Yuan S, Kok KH, To KK, Chu H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395 (2020): 514-523.
- Kreutz R, Algharably EA, Azizi M, Dobrowolski P, Guzik T, et al. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19European Society of Hypertension COVID-19 Task Force Review of Evidence. Cardiovascular Research (2020).
- Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacological research 125 (2017): 57-71.
- Ingelfinger JR. Angiotensin-converting enzyme 2: implications for blood pressure and kidney disease. Current opinion in nephrology and hypertension 18 (2009): 79-84.
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Annals of Hematology (2020): 1-4.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 323 (2020): 1061-1069.
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. Epub ahead of print (2020).
- Fan BE, Chong VC, Chan SS, Lim GH, Lim KG, et al. Hematologic parameters in patients with COVID-19 infection. American journal of hematology (2020): E131-E134.
- Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Annals of Hematology (2020): 1.
- Zhu J, Ji P, Pang J, Zhong Z, Li H, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. Journal of Medical Virology (2020).
- Zhou Y, Fu B, Zheng X, Wang D, Zhao C, et Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. BioRxiv (2020).
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544 (2017): 105-109.
- Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Annals of internal medicine 160 (2014): 389-397.
- Iba T, Levy JH, Wada H, Thachil J, Warkentin TE, et al. Subcommittee on Disseminated Intravascular Coagulation. Differential diagnoses for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH. Journal of Thrombosis and Haemostasis 17 (2019): 415-419.
- Klok FA, Kruip MJ, Van der Meer NJ, Arbous MS, Gommers DA, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis research (2020).
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis 18 (2020): 844-847.
- Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood, The Journal of the American Society of Hematology 135 (2020): 2033-2040.
- Rico-Mesa JS, Rosas D, Ahmadian-Tehrani A, White A, Anderson AS, et al. The role of anticoagulation in COVID-19-induced hypercoagulability. Current cardiology reports 22 (2020): 1-6.
- Onofrio L, Caraglia M, Facchini G, Margherita V, Placido SD, et al. Toll-like receptors and COVID-19: a two-faced story with an exciting ending (2020): FSO605.
- Mishra KP, Singh AK, Singh SB. Hyperinflammation and immune response generation in COVID-19. Neuroimmunomodulation (2020): 1-7.
- Wong RS. Inflammation in COVID-19: from pathogenesis to treatment. International journal of clinical and experimental pathology 14 (2021): 831.
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature reviews immunology 20 (2020): 355-362.


 Impact Factor: * 3.5
Impact Factor: * 3.5 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 14.80%
Acceptance Rate: 14.80%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 