Somatic Cell-Fusion and/or Co-Culturing: A Mechanism for Cell Re-Programming Prior to Cell Therapy of Neurodegenerative Diseases
Article Information
Ashok Chakraborty*, Anil Diwan
Allexcel, Inc, CT, USA 1, Controls Drive, Shelton, CT 06484, USA
*Corresponding author: Ashok Chakraborty, Allexcel, Inc, CT, USA 1, Controls Drive, Shelton, CT 06484, USA
Received: 14 December 2022; Accepted: 22 December 2022; Published: 13 January 2023
Citation: Ashok Chakraborty, Anil Diwan. Somatic Cell-Fusion and/or Co-Culturing: A Mechanism for Cell Re-Programming Prior to Cell Therapy of Neurodegenerative Diseases. Fortune Journal of Health Sciences 6 (2023): 18-30.
View / Download Pdf Share at FacebookAbstract
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the two most prevalent neurodegenerative diseases which generally start after 50-55 ages of life where the brain neural cell gets destroyed. Transplantation of the dopaminergic (DA-ergic) neuronal cells, though, could help the diseases but the most obstacle is the availability of a sufficient number of such cells for replacement therapy. Neural stem cells (NSCs) are able to produce DA-ergic neurons and also have the capacity to act as a useful vehicle for genetic and molecular therapies within the central system (CNS), however, the sustainability of slowly and senescence NSCs must be ensured through genetic manipulation both in vitro and in vivo. NSCs grow very occurs after a few passages. Here we'll discuss many options to modify the NSC cells for a better growth, and increased survival length, as well as their ability to control the release of dopamine within the neural synaptic cleft. Cell-Cell interactions in terms of co-culturing or cell fusion are commonly known to change the cells’ fate by genetic reprogramming, and thus discussed here for evaluation of their use in the transplantation process.
Keywords
Neurodegenerative Diseases, Cell therapy, Cell-fusion, Gene reprogramming
Article Details
3. Introduction
Cell replacement therapy can be best described as the replenishment of cell loss by transplantation of a new cell of that type. The cell to be transplanted should be either genetically modified or modified by cell-cell interaction to create a modified cell type for the desired activities. Here we'll discuss neural cell reprogramming within the scenario of cell replacement therapy for neurodegenerative diseases where active cell loss is prominent. Alzheimer’s disease (AD) and Parkinson’s disease (PD), which generally starts in the mid-age of life and shows the loss of neural dopaminergic (DA-ergic) cells within the hippocampus and neural synaptic cleft at the Substantia nigra (SN), respectively [1, 2]. Supplementation of dopamine, a neural cell-derived product, from can ameliorate the disease symptoms temporarily, however, long time treatment with this neurotransmitter may cause motor neuron defect, dyskinesia etc. [3, 4]. Therefore, a controlled release of dopamine may be possible only by transplantation of the DA-ergic neural cells in there [5]. The biggest obstacle in such therapy of AD and PD, is the availability of the right cell type in sufficient amounts. Most of the cells, which may come on the list, have various negativities like the formation of teratomas in the future, or may differentiate to other cells. Besides, immune rejection, moral issues of using those cells, and availability in sufficient amounts for transplantation, are the added obstacles. Therefore, the selection of isogenic cells and reprogramming them to a differentiated neural cell may be the most effective thought for cell preparation for therapeutic purposes [6].
2. Concept of Cell Reprogramming
Cell reprogramming concepts have been classically developed in the fields of developmental and stem cell biology and are currently being explored for regenerative medicine, given their potential to generate desired cell types for replacement therapy. Cell reprogramming refers to the ability to redefine the identity of a cell by changing its epigenetic and transcriptional landscapes, reflected in the acquisition of new morphological, molecular, and functional features [7]. These changes entail a complete reversion of cell fate or modification of somatic cellular identity.
Somatic cells can be reprogrammed to pluripotency, acquiring self-renewal and pluripotent features similar to embryonic stem cells (ESCs) [8]. Alternatively, lineage reprogramming involves the conversion of specialized cells into a different somatic cell type without transiting through pluripotency [9]. This process can occur directly (transdifferentiation or direct cell reprogramming) or progress through an intermediate progenitor state that re-differentiates into different cell types.
The discovery of iPSCs now becoming a hope to replace the cell loss caused by the disease [8-10]. In fact, somatic cells could be reprogrammed into induced pluripotent stem cells (iPSCs) and these cells could be used to model Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD), and Parkinson’s disease (PD) [11-14]. The foremost promising reprogramming technique for the generation of induced pluripotent stem cells (iPSCs) is the transduction of defined transcription factors like OCT4, SOX2, and KLF4 [8, 15, 16]. In general, the efficiency of iPSCs generation is extremely low, and also reprogrammed clones often show differences in the amount of the epigenome and transcriptome compared with the stem cells derived from the embryos [17-19].
3. Reprogramming using somatic cell nuclear transfer (SCNT)
The differentiated state of a somatic cell may be reversed experimentally to that of another cell type by a process termed as somatic cell nuclear transfer (SCNT) [20]. SCNT enables the direct generation of organisms from single donor cells. Although both the SCNT and the iPSC technologies can reprogram differentiated somatic cells into cells of embryonic state, the paths to achieve pluripotency are likely different. SCNT reprogramming is very fast, within an hour [21, 22], whereas the establishment of a stable iPSC takes several days to weeks to establish a stable cell. Furthermore, SCNT reprogramming, at least for chromatin accessibility and transcriptome reprogramming, is much more efficient than that of iPSCs [19].
4. Co-culturing of Cells Induces Cellular Behavior
Co-culturing of two different types of cells give the opportunity to go for cell-cell interaction, which may lead to going for the generation of a new cell type with altered functions as well as altered capabilities [23-25]. Many such references are available in the literature with somatic cells, cancer cells, etc. [25]. Proliferation and differentiation of NSCs to neurons were induced when co-cultured with glioblastoma cells (Wang et. al. 2010). Cell survival and neuronal differentiation of transplanted NSCs noticeably improved in the ischemic striatum of the middle cerebral artery occlusion (MCAO) rat model system [27]. Sertoli cells (SCs) secreted neurotropic factors, and co-culturing with NSCs showed neurite outgrowths [28].
Microglia can stimulate the proliferation of NSCs in culture, increase the release of mitogenic factors, promotes differentiation to neurons, and also the formation of oligodendrocytes. Bone marrow-derived mesenchymal stem cells (BM-MSCs) that release chemokine ligand-2 (CCL-2) stimulate proliferation and differentiation of NSCs and also can protect the later cells from neurotoxic effect of 6-hydroxy dopamine [29, 30]. Olfactory ensheathing cells (OECs) are specialized glial cells that have properties of both SCs and astrocytes [31]. They have been shown to promote the differentiation of NSCs proliferation in co-culture [26], although not all astrocytes do so [27]. We showed before that hNSCs produce increased amounts of BDNF, GDNF, and Dopamine (DA) when co-cultured with human normal melanocytes (hNMCs) [32].
5. Cell Fusion and Reprogramming may beStudied with Rigor in Cell Culture System
In one instance, hybrid cells were generated when mouse bone marrow cells were grown on mitotically-inactive fibroblast feeder cells. These hybrids contained genetic markers from both cell types and expressed endothelial markers when plated on a matrigel matrix [33]. In another study, neurosphere cells derived from embryonic day 14.5 mouse forebrain spontaneously fused with ESCs under conditions of co-culture [34]. The fused cells had markers from both fusion partners, grew with ESC-like morphology, and contained a tetraploid complement of chromosomes. These neurosphere/ESC hybrids contributed readily to chimeras. Spontaneous cell fusion was also detected when hygromycin-sensitive mouse ESCs were co-cultured with hygromycin-resistant primary murine brain cells for five days [35]. The resulting hybrid cells formed hygromycin-resistant colonies and expressed the stem-cell-specific Foxd3 gene from the chromosome originally present within the neural cells. Furthermore, these hybrid cells contributed to all or any three germ layers in chimeras. Spontaneous fusion of cancer cells with the host cells form a hybrid cell as shown in murine models similar to human in vivo, and people hybrids present host cell marker genes and showed an increased metastatic potential in them [35-38].
6. Cell-Cell Fusion as a Method of Cell Reprogramming
Another major technique of reprogramming is a cell-cell fusion that had been reported earlier between somatic cells and hESCs [39, 40]. In brief, this method allows the melding of two or more cells into one cell called a heterokaryon (or homokaryon, if fusion occurs between identical cell types), and this state persists for two to three days, after which the nuclei fuse and produce a hybrid cell. This hybrid cell contains a single polyploid/tetraploid nucleus capable of reentering cell division [41-43]. In particular, fusogens are mandatory for the contribution to the steric formation of several cell fusion-related lipid intermediates named "the hallmarks of cell-cell fusion" [44]. In one study, hybrids were generated by a fusion of male murine Hprt-/- ESCs with female mouse splenocytes [45]. Fused cells were selected in the HAT medium. The resulting hybrid cells exhibited near-tetraploid karyotypes and formed a variety of embryonic bodies containing cell types corresponding to all three germ layers [46]. In addition, these cells contained synchronously replicating X chromosomes, suggesting that the inactive X chromosome of splenocyte origin was reprogrammed to a pre-inactivation state. In another study, a fusion of thymocytes from an adult female mouse, harboring a silent GFP transgene, with ESCs from a male mouse yielded hybrids that expressed GFP [47]. The thymocyte/ ESC hybrids contained reactivated X chromosome of thymocyte origin as judged by fluorescent in situ hybridization for Xist RNA. The pluripotency factor nanog is expressed in morula, blastocyst stage embryos, and in ESCs, but not in differentiated somatic cells. The silent nanog gene, however, can be reactivated upon the fusion of somatic cells with ESCs or when somatic nuclei are transplanted into oocytes [48]. Cell–Cell fusion-mediated reprogramming is a faster and more efficient method than the iPSCs method, because the hESC used for cell fusion provides all the factors required for the maintenance of pluripotency. However, the fused and reprogrammed cells retain both the somatic and ES cell genomes, a visible barrier to their application in anything apart from mechanistic studies. Cell fusion is one of all the several approaches that allow differentiated cells to return to a pluripotent state [49-51]. Cell fusion is a very important physiological process that is required for discrete events in vivo like fertilization, tissue repair, and immune defense [52]. Outside these settings, cell-to-cell fusion is comparatively uncommon, but will be induced experimentally, for instance, using agents like inactivated Sendai virus [53], polyethylene glycol (PEG) [54], or electrical charge, in an ex vivo setting. Cell fusion hybrids between mouse ESCs and human fibroblasts are often identified as each of the parental nuclei may be distinguished and monitored. Cell types may be labeled with different fluorescent tags before cell fusion and flow cytometry is accustomed to select the heterokaryons that are formed (Villafranca et. al. 2020), additionally as following how they alter over time.
7. Fusion-Mediated Reprogramming Using Other Embryonic Cells
Primordial germ cells (PGCs) contain interesting reprogramming activities since they undergo imprint erasure shortly after migration to the genital ridge but before the establishment of male or female gonadal fates. PGCs are identified by the expression of an Oct4-GFP transgene, which allows their purification by fluorescence-activated cell sorting (FACS). Analysis of DNA contained within PGCs prepared in this way revealed demethylation at multiple genetic loci including both imprinted and non-imprinted genes [55, 56]. This finding makes PGC cells and their cultured derivatives, embryonic germ cells (EGCs), attractive candidates for intentional fusion with somatic cells to cause reprogramming. Indeed, when PGCs marked with ROSA26βgeo were electro-fused to thymocytes, Several imprinted and non-imprinted genes underwent demethylation, adopting a state observed in normally-derived EGCs [47]. In overall morphology, the hybrids resembled EGCs but inspection of the hybrid karyotype revealed a tetraploid complement of chromosomes. When EGC/T-cell hybrids were injected into host blastocysts and implanted into pseudo-pregnant mothers, β-galactosidase expression was widely observed in chimeric embryos on days between 9 and 10. Though EGCs contain potent demethylating activities, it remains unclear if the expected loss of imprints in PGC/somatic fusions would have consequences for cell-based therapies.
8. Mouse Embryonic Carcinoma Cells (ECCs) Can Reprogram Somatic Cells
Human T- lymphoma cells could be reprogrammed upon fusion with mouse embryonic carcinoma cells. During this instance, the hybrids expressed human Oct4 and Sox-2 genes, showing that human genes involved in pluripotency were de-repressed by the hybridization event. In addition, the CD45 surface protein was reduced in ECC/T-lymphoma hybrids. This suggested an alteration of lymphocytic characteristics in the hybrids. A comparison of different reprogramming methods is shown in Table 1.
Table 1: Comparison of Different Reprogramming Methods
|
Queries |
Cell-Cell Fusion |
Transduction of transcription factors |
Somatic Cell Nuclear Transfer (SCNT) |
|
Mechanisms |
The melding of two or more cells into one cell called a heterokaryon [49, 57] |
Reprogramming factors, OCT4, SOX2, KLF4, c-MYC, NANOG, and LIN-28 were transiently transfected simultaneously with the nuclear-localized ECFP (ECFP-nuc) expression vector into the OGIH HDFf or MRC5 [42, 43] |
SCNT is a technique by which the nucleus of a differentiated cell is introduced into an oocyte from which its genetic material has been removed by a process called enucleation [62] |
|
Efficiency |
hESC cytoplast fusion could initiate reprogramming but was never able to complete reprogramming [58]. |
Enucleated hESC-fusion initiates reprogramming but does not yield completely reprogrammed cells [58] |
The cloning efficiency is varying within relatively low values between 0.5% and 5% offspring per transferred SCNT embryos [63]. |
|
Viability of the Processes |
Cell fusion provides relatively efficient reprogramming [58, 59]. |
Nuclear transfer, reprogramming through direct introduction of a somatic nucleus into the environment of a pluripotent cell provides relatively efficient reprogramming [58, 61] |
Low efficiency in creating normal viable offspring in animals by SCNT (1–5%) and the high number of abnormalities in these cloned animals is due to epigenetic reprogramming failure [64] |
|
Merits/Demerits |
Cell-Cell fusion methods takes less than 10 days to make the hybrids and efficiency is more than 0.005% [60] |
High throughput single-cell multi-omics methods are potential to understand the transcription factors and chromatin changes necessary for cell type conversion [59] |
Ability to confirm in vitro the desired genetic modification in the somatic cells prior to animal production [65] |
|
Identify the earliest events in reprogramming, and enable to distinguish the stages before and after cell division [49] |
iPSC generation is very low and reprogrammed clones often show differences at the level of the epigenome and transcriptome when compared with stem cells derived from embryos and the efficiency was less than 0.001% [43] The reprogramming process of iPSC generation required more than 4 weeks for emergence of hESC-like colonies, and the efficiency was less than 0.001% [9, 15, 61] |
There are ethical and practical barriers to apply in humans [65] |
|
|
Reprogramming by cell fusion with hESC is much more efficient and faster than virus transduction of reprogramming factors [43] |
|
||
|
Fusion yielded almost no partially reprogrammed cell colonies. However, the fused cells were tetraploid or aneuploidy [58, 61] |
Such a comparison would indicate whether cytoplast fusion is a feasible alternative to iPS cell generation, and would provide some insight into how transduction of a few key pluripotency factors compares with provision of the complete pluripotent cytoplasmic or cytoplasmic plus nuclear environment in cell reprogramming. The cell fusion system also offers an opportunity to explore gene reactivation and silencing which is useful in the context of designing strategies to reverse certain disease states. The goal of regenerative medicine is to restore form and function to damaged tissues. While the fused cells are unlikely to be directly suitable for medical use, they can offer an important experimental tool to examine the pathways of cell conversion, genome repurposing, and locus reactivation, which can be imitated for therapeutic benefit.
9. Polyethylene Glycol (PEG)-Mediated Cell Fusion
Cell fusion may therefore be considered an efficient method of reprogramming where the hESCs partner provides a completely functional network of pluripotency factors and cell signaling molecules. However, fully reprogrammed colonies appeared in cell fusion-mediated reprogramming much faster than in four-factor-mediated iPSCs generation [66, 67]. Much PEG-mediated fusion reprogramming were reported before as mouse ESCs/iPSCs were fused with neurospheres cumulus cells, splenocytes, mesenchymal stem cells (MSCs), and mouse embryonic fibroblasts (mEFs) [46, 68-70]. Human ESCs are PEG-fused to fibroblasts and myeloid precursors [39, 40, 43]. In cancer biology, PEG-mediated fusion between non metastatic melanoma cells with macrophages reprogrammed to metastatic cancer cells [71-73]. Further, mouse non-metastatic melanoma cells when fused with human leukocytes expressed human genes within the hybrid together with their increased metastatic potential [71].
10. Spontaneous Fusion
Interest was stirred within the field of adult somatic cell research when engraftments of marked bone marrow stem cells (BMSCs) displayed remarkable lineage plasticity following engraftment. However, subsequent studies demonstrated that the fusion of ESCs and somatic cells occurs spontaneously under conditions of co-culture [34, 75]. These findings suggested alternative interpretations during which endogenous somatic cells acquired somatic cell markers through simple fusion events. Though these findings raised concerns, later studies that controlled for cell fusion revealed that adult stem cells do possess a stimulating degree of developmental plasticity. For example, bone marrow-derived cells possessed the power to turn out to be cells with epithelial character, apparently without fusion, as judged by a sensitive crelox strategy to detect fusion events [54]. Taken together, these results indicate a promising future for investigations of adult vegetative cell plasticity, but such research should be conducted in conjunction with robust methods to detect fusion events.
11. The Future of Cell Reprogramming for Cell Therapy of Neurodegenerative Diseases
Different types of reprogramming can result in producing undifferentiated cells with varying degrees of “stemness”. Technically, somatic cell nuclear transfer (SCNT) is the only method that can reprogram a somatic cell into a totipotent cell capable of creating a complete organism. However, as described above, cellular reprogramming methods usually involve induced pluripotency via in vivo or in vitro manipulation, which is expensive, time- consuming, and involves cumbersome techniques. Finding a way to produce iPS cells that are near “biological equivalent” embryonic stem cells is of interest to the research community because this would allow iPS cells to be created from patients for immediate use in transplantation. For many medical conditions, this is a difficult goal, because a patient’s cells have often been affected by their own disease. A non-genetic and PEG-mediated cell fusion of DA-ergic neural cells with other selectively chosen partner cells (based on research, and also see the recent review) which is our current research of interest to improve the growth potential and differentiation of the NSCs may remove the need for immunosuppressive drugs and eliminate the rejection of transplanted cells.
12. Evidence of Using NSCs for PD and AD Cell Therapy
NSCs may provide a virtually unlimited sources of self-renewing progenitors for transplantation. The potential applications and technical challenges of this approach have been critically reviewed [76]. It has been reported that transplanting differentiated monkey embryonic NSCs into the monkey putamen leads to their proliferation into fully functional DA neurons [77]. Implantation of these DA neurons caused sustained improvement of MPTP-induced motor symptoms that was significant over a 10 –14-week follow-up period compared with a sham group. Proliferation into fully differentiated DA neurons has been observed following the implantation of undifferentiated human NSCs into MPTP-lesioned monkeys [78].
13. Progress in Dopaminergic Cell Replacement and Regenerative Strategies for Parkinson's Disease [79]
Understanding of Parkinson's disease therapy through the use of cell reprogramming becomes evident. Interestingly, direct reprogramming using just one pluripotent factor can generate expandable stem/progenitor cells [80-83]. Recently, human iNPCs (hiNPCs) have been successfully differentiated into the motor and dopaminergic neurons using specific patterning molecules [84, 85] transplanted mouse iNSCs into the brains of toxin-induced mouse models of PD. Restoration of brain tissue led to enhanced functional recovery of the animals in behavioral assessment. Another study investigating the effects of iNSC engraftment in the 6-OHDA-induced mouse model of PD found restored dopamine production and improved motor behavior, despite low survival rates of engrafted cells [86]. Transplantation of mouse iNSCs into the hippocampus of the APP/PS1 mouse model of AD can significantly improve the spatial learning and memory of APP/PS1 AD mice [87]. In cynomolgus monkey, iPSC-derived dopaminergic neurons were transplanted into the putamen of a non-human primate Parkinsonian brain. The reprogrammed neurons survived and underwent extensive outgrowth into the transplantation site and surrounding putamen; improved motor function and increased motor activity without immune suppression [88]. The transplantation of these neurons into the rat striatum lesioned by 6-OHDA, a functional model of PD, successfully demonstrated improvements in motor function post-neuron engraftment [89].
14. DA-ergic Neuron-based Replacement Therapy for AD
Similarities among Alzheimer’s disease, Parkinson’s disease, and Dementia may call for a similar treatment [90-97]. In fact, in mouse AD models, transplantation of NSCs was reported to improve cognition function mediated by the neurotropic factor BDNF [97-99]. Further, transplantation of growth factor-secreting NSCs increased neurogenesis and cognitive function in a rodent AD model [98], and aged primate brains [100]. Other recent AD rodent model studies have reported that NSC transplantation decreased neuroinflammation [101], attenuation of tau and Aβ in AD neuropathology [102], promotion of neurogenesis and synaptogenesis [103, 104], and reversal of cognitive deficits [101, 103, 104].
Dopamine therapy improves cognitive function in Alzheimer’s disease. The study is supported by the Alzheimer's Drug Discovery Foundation and published in JAMA Network Open. The study provides the first evidence that “rotigotine”, a drug that acts on dopamine transmission in the brain, improves cognitive function in Alzheimer's disease [105]. In brief, hNSCs being are equipped with both Tyrosine hydroxylase, a key rate-limiting enzyme for Dopamine production, moreover, as its scavenging enzymes (DAT and MAO-B) which might efficiently control the physiologic level of that neurotransmitter [106]. hNSCs become the primary choice for cell replacement therapy. Furthermore, hNSCs can produce brain derived neural factors (BDNF) and glial-cell-derived neural factors (GDNF) which might influence the expansion and Dopamine production ability of hNSCs in an autocrine manner [107-109]. While the therapeutic mechanisms behind these changes are not yet fully understood, they are likely mediated by both the paracrine release of neuro-protective or immune modulatory factors [93], and by direct neuronal differentiation [110, 111], although the widespread generation of non-neuronal glial cell types from transplanted NSCs remains a major limiting factor for neuro-replacement strategies [111]. A modified neural cell, after reprogramming by any of the above-mentioned methods, may well be used for transplantation within the brain for cell therapy. Since spontaneous PEG-mediated fusion or co-culturing-mediated reprogramming showed plenty of advantages over genetic or virus-mediated reprogramming, we will keep hope for the long-run development of the therapeutic achievement for AD/PD cell therapy.
15. Other Importance of Using NSCs for Cell Therapy of Neurodegenerative Diseases
NSCs can also act as a vehicle or career of genetic material. NSCs stably transduced with human nerve growth factor genes survive and integrate into the cerebral cortex of AD animal model (Rat) upon transplantation and enhance cognitive performance. This survival and integration were not observed in the same rat model transplanted with NSCs without genetic modification [112, 113]. Transplantation of NSCs is also used as a vehicle to deliver potential therapeutic agents, including neprilysin, insulin-degrading enzyme, plasmin, and cathepsin B, to decrease Aβ levels in AD mouse models [114]. Neural stem cells (hNSCs) can be an efficient candidate to deliver neurotropic factors or enhance gene expression to modify the course of the disease [115-117]. These cells also have been considered for use in cell replacement therapies in various neurodegenerative diseases, as well as in other brain-related diseases such as ischaemic and neoplastic lesions. Here, we speculate on ways in which neural stem cells [118].
16. Limitations of Somatic Cell Fusion Reprogramming Methods for Cell Therapy
Although the fusion hybrid cells show pluripotential characteristics, the fusion hybrid cells are not identical to the pluripotent fusion partner cells. The fusion hybrid cells can form chimera but not contribute to the germline. Although fusion-induced reprogramming is very efficient (about 95%), the resultant hybrid cells lack therapeutic potential because of their tetraploidy and the presence of exogenous genes from the pluripotent fusion partner cells [119].
A critical issue for bringing iPSCs into the presetting clinic is the non-sufficient scale and preservation of neurons. Cryopreservation of a large number of cells will interfere with this challenge. Functional and non-tumorigenic dopaminergic neurons lost with extensive fiber innervation in the midbrain of PD can be retained with regenerative medicine and iPSCs derived dopaminergic neurons. It seems not only the donor cells but also the host niche is important for accepted transplantation [120], PD is multiple neuronal subtype damage such as midbrain dopaminergic neurons. Dopamine agonist treatment side effects open a new therapeutic strategy based on stem cells [121]. Recently, in a study, the researchers created a library of iPSCs from Parkinson’s disease as a useful source to focus on common and divergent pathogenic mechanisms of neurodegenerative disorders [122]. Wide and practical iPSCs studies of PD treatment, establish a new alternative to knowing and treat it deeply. Further, due to the number of divisions that the cells underwent, there are difficulties in the conservation of genomic instability. Finally, we know that no developed technology is perfect, but it is possible to eliminate the negativities in our hands by continuing to progress [123].
17. Challenges with Clinical Translation and Potential Solutions
There are several major difficulties associated with using trans-differentiated cells in clinical applications. The most glaring issue is the use of lentiviruses to infect cells, due to the small possibility of unintended insertional mutagenesis [124]. These mutations, while unlikely, could cause drastic, unforeseen consequences in the host, such as the emergence of cancer [125]. Understandably, many government agencies take precautions due to this risk. Non-integrating viruses and other methods that do not integrate DNA into the host genome do not pose these threats but have much lower reprogramming efficiencies. Therefore, there is a need to efficiently transdifferentiate cells while avoiding the possibility of mutagenesis.
The advent of dCas9 allows for a drastic reduction of the chance of mutagenesis through its ability to multiplex. When lentiviral vectors are used to overexpress multiple exogenous transcription factors, more than one vector may be used due to the cargo capacity limitations of lentiviruses. However, trans-differentiation methods utilizing dCas9 only need to use one vector to efficiently express the dCas9. Once the cells express dCas9, several gRNAs targeting various genes can be added through non-integrating methods, allowing the dCas9 to regulate the expression of several genes despite the cells receiving a single lentivirus infection [126].
Thus, reducing the number of DNA-integrating viruses needed to trans-differentiate cells lowers the chance for insertional mutagenesis. Another alternative that would completely remove the potential for mutagenesis would be through the delivery of dCas9/ gRNAs ribonucleoprotein complexes (dCas9 RNPs). dCas9 RNPs consist of dCas9 preloaded with gRNAs, which are then directly delivered to cells using electroporation or transfection techniques, eliminating the need for DNA integration into the genome. However, dCas9 RNPs come with a major drawback; they are cleared rapidly from the cell through protein degradation pathways [127]. Therefore, the dCas9 RNPs would need to be re-introduced into the source cells at regular intervals to effectively trans-differentiate the cells.
Another concern with trans-differentiated cells is their ability to completely mimic their desired cell phenotype, as it is likely that the trans-differentiated cells will not be identical to their native counter parts. Thus, more complete reprogramming processes are needed to generate trans-differentiated cells that more closely resemble the desired cell phenotypes. Through thorough testing and experimentation, the major characteristics of the reprogrammed cells can be analyzed and compared to native cells. Although in vitro assays will analyze some of the reprogrammed cells’ properties, well-designed in vivo assays are necessary to fully characterize them in a physiological setting. Current in vivo studies are superficial and typically fail to detail more than a handful of reprogrammed cell capabilities; as such, more extensive testing in animal models is necessary before trans-differentiated cells see any translation to clinical applications [128, 129]. Lastly, reprogramming efficiency is another problem associated with the trans-differentiation process [128]. A low conversion efficiency generally leads to a lengthy period of time before there are enough reprogrammed cells for any clinical application, hindering the use of trans-differentiated cells in humans, as clinical situations are often time-sensitive [130]. Consequently, improving the efficiency and cell yield of the transdifferentiation process is vital to make the trans-differentiation more favorable for clinical applications. This can be done with a myriad of methods, which include optimizing biochemical [131], biophysical [132], and biomechanical [133] cues the cells experience during the reprogramming process, targeting additional transcription factors, and transitioning from exogenous overexpression to endogenous up-regulation via dCas9. Here we display a table showing some advantages and disadvantages of using stem cells in neurodegenerative cell therapies (Table 2). Each stem cell has a specific neurogenic potential and can achieve certain results, but there are still many problems to be solved before they can be used for clinical applications.
Table 2: Advantages and Disadvantages of Stem Cells
|
Cells |
Sources |
Advantages |
Disadvantages |
|
Neural stem cells (NSCs) |
Primary tissues, (fetal, neonatal, and adult brain) Embryonic stem cells Induced pluripotent stem cells |
Easy to access No ethical issues (but based on sources) No histocompatibility |
Strong immunogenicity (but based on sources) The mechanism of cell proliferation, differentiation, and migration is unclear |
|
Mesenchymal stem cells (MSCs) |
Bone marrow, Adipose tissue, and Umbilical cord |
Widespread sources Secrete multiple bioactive factors Directional migration |
Bone marrow mesenchymal stem cells-limited raw materials Poor proliferation, and traumatic No unified identification standard for umbilical cord blood mesenchymal stem cells, and the culture technology in vitro and differentiation are not yet mature |
|
Embryonic stem cells (ESCs) |
Early embryo |
Strong proliferation ability Abundant sources Can be passed on |
Ethical issues The allograft produces a great rejection reaction Unrestrained differentiation Tumorigenicity |
|
Induced pluripotent stem cells (iPCSs) |
Gene recombination |
No ethical issues No histocompatibility |
Complex operation process Low reprogramming efficiency Mutation induction Tumorigenicity |
18. Conclusions
Recent experiments that make use of spontaneous or intentional fusions of somatic cells with ESCs, ECCs, EGCs, or others, have shown that somatic nuclei can even be reprogrammed through cell fusion. Such reprogramming consists of the epigenetic remodeling of the somatic genome to yield a new (and typically more embryonic) state of chromatin organization and organic phenomenon. Performing assays of developmental potential like chimera formation can operationally assess the reprogramming of somatic/embryonic cell hybrids.
Three conditions ought to be satisfied for proof of reprogramming in hybrid cells:
(1) The organic phenomenon in hybrids must carry with it the sum of individual patterns of organic phenomenon present in unfused parental cells.
(2) Gene silencing or activation in hybrids is best demonstrated with polymorphisms that mark the "parental" origin of reprogrammed organic phenomena.
(3) Reprogrammed hybrid cells should exhibit unique developmental potential or biological characteristics.
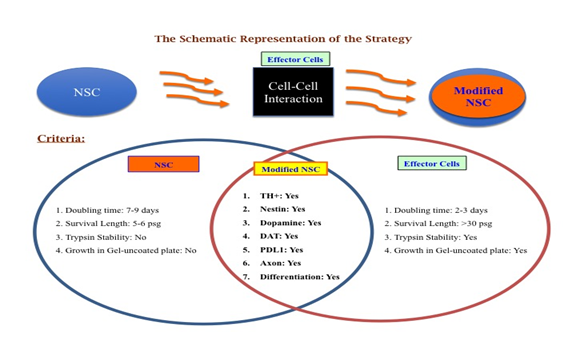
Trans-differentiation is a powerful tool for generating functional cell phenotypes without the need for iPSCs or embryonic stem cells. Over the past several years, several techniques for cellular reprogramming have been developed and various targeted cell phenotypes have been generated, with encouraging results. Although current trans-differentiation methods are somewhat limited due to efficiency problems, there is ongoing research that aims to improve efficiency and there have been preliminary success with the emergence of dCas9 as an alternative to transgene overexpression methods. Regardless of efficiency limitations, a wide array of cells has been successfully generated and their ability to mimic physiological cells shows great promise, especially with the advent of trans-differentiating cells in situ. These cells still have a long way to go to achieve fully-functional states and see use in tissue engineering, as rigorous clinical testing needs to be conducted. Nevertheless, considering how infantile the fields of reprogramming and trans-differentiation are, it would not be surprising to see transdifferentiated cells have a place in personalized regenerative medicine and tissue engineering in the future [128]. This article is a review of our concept on the idea whether and how a cell can be modified for better efficacy in terms of Dopamine production and cell survival and can bypass the genetic defect in the host for PD therapy, but not on any Experimental Observations. A schematic diagram-1 is posted here to view the concept of our future strategy. Choice of effector cells is matter of selection based on the goals [6].
Acknowledgments
We acknowledge all our colleagues for their help during the preparation of the manuscript by providing all the relevant information. Thanks to Ms. Bethany Pond for her Editorial assistance.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author’s Contribution
Both the authors have contributed equally to preparing this article, reading, and approving the final manuscript.
Conflict of Interests
This research is supported by internal grant from All Excel, Inc. The authors report no conflicts of interest.
Consent for Publications
Both the authors have agreed to submit this paper for publication.
Ethical Approval
Not applicable
References
- Nobili A, Latagliata E, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun 8 (2017): 14727.
- Martorana A and Koch G. Is dopamine involved in Alzheimer's disease? Frontiers in Aging Neurosciences 6 (2014): 1-7.
- Dorszewska J, Prendecki M, Lianeri M, et. al. Molecular Effects of L-dopa Therapy in Parkinson's Disease. Curr Genomics 15 (2014): 11-17.
- Marsden CD. Problems with long-term levodopa therapy for Parkinson's disease. Clin Neuropharmacol 17 (2014): S32-44.
- Vishwakarma SK, Bardia A, Tiwari SK, et. al. 2014. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J Adv Res 5 (2014): 277-294.
- Chakraborty A, Diwan A. Selection of Cells for Parkinson's Disease Cell-Therapy. Int. J Stem Cell Res Ther 6 (2019): 063-083.
- Pereira CF, Lemischka IR, Moore K. Reprogramming cell fates: insights from combinatorial approaches. Ann N Y Acad Sci 1266 (2012): 7–17.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors Cell 126 (2006): 663–76.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131 (2007): 861–72.
- Takahashi K & Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nature Reviews Molecular Cell Biology 17 (2016): 183–193.
- Smith DK, He M, Zhang C-L, et al. The therapeutic potential of cell identity reprogramming for the treatment of aging-related neurodegenerative disorders. Prog Neurobiol 157 (2017): 212-229.
- Doi D, Samata B, Katsukawa M, et al. 2014. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports 2 (2014): 337-50.
- Kim H-S, Kim J, Jo Y, et al. 2014. Direct lineage reprogramming of mouse fibroblasts to functional midbrain dopaminergic neuronal progenitors. Stem Cell Research 12 (2014): 60-68.
- Tian C, Li Y, Huang Y, et al. Selective Generation of Dopaminergic Precursors from Mouse Fibroblasts by Direct Lineage Conversion. Sci Rep 5 (2015): 12622.
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318 (2007): 1917–1920.
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 448 (2007): 313–317.
- Chin MH, Mason MJ, Xie W, et al. 2009. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5 (2009): 111–123.
- Riggs JW, Barrilleaux BL, Varlakhanova N, et al. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev 22 (2013): 37-50.
- Doi A, Park IH, Wen B, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 41 (2009): 1350–1353.
- Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science 322 (2008): 1811-5.
- Djekidel MN, Inoue A, Matoba S, et al. Reprogramming of chromatin accessibility in somatic cell nuclear transfer is DNA replication independent. Cell Rep 23 (2018): 1939-1947.
- Egli D, Chen AE, Saphier G, et al. Reprogramming within hours following nuclear transfer into mouse but not human zygotes. Nat. Commun 2 (2011): 488.
- Wu X, Wang S, Li M, et al. Conditional reprogramming: next generation cell culture, Acta Pharmaceutica Sinica B 10 (2011): 1360-1381.
- Liu X, Meyers C, Schlegel R, et al. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest 120 (2010): 2619-2626.
- Vis MAM, Ito K and Hofmann S. Impact of Culture Medium on Cellular Interactions in vitro Co-culture Systems. Front. Bioeng. Biotechnol 8 (2020): 911.
- Wang G, Ao Q, Gong K, et al. Synergistic effect of neural stem cells and olfactory ensheathing cells on repair of adult rat spinal cord injury. Cell Transplantation 19 (2010): 1325–1337.
- Luo L, Guo K, Fan W, et al. Niche astrocytes promote the survival, proliferation and neuronal differentiation of co-transplanted neural stem cells following ischemic stroke in rats. Exp. and Ther Med 13 (2017): 645–650.
- Shi B, Deng L, Shi X, et al. The enhancement of neural stem cell survival and growth by co-culturing with expanded Sertoli cells in vitro. Biotechnol Prog 28 (2012): 196-205.
- Habisch HJ, Liebau S, Lenk T, et al. Neuroectodermally converted human mesenchymal stromal cells provide cytoprotective effects on neural stem cells and inhibit their glial differentiation. Cytotherapy 12 (2010): 491–504.
- Lee H, Kang JE, Lee JK, et al. Bone-marrow-derived mesenchymal stem cells promote proliferation and neuronal differentiation of Niemann–Pick type C mouse neural stem cells by upregulation and secretion of CCL2. Human Gene Therapy 24 (2013): 655–669.
- Gudiño-Cabrera G, Nieto-Sampedro M. Schwann-like macroglia in adult rat brain. Glia 30 (2000): 49-63.
- Chakraborty AK and Diwan A. Coculturing NSCs with Melanocyte Increased its Dopamine and Neural Factor Secretion. Acta Scientific Neurology 4 (2021): 70-78.
- Que J, El Oakley RM, Salto-Tellez M, et al. Generation of hybrid cell lines with endothelial potential from spontaneous fusion of adult bone marrow cells with embryonic fibroblast feeder. In Vitro Cell Dev Biol Anim 40 (2004): 143–9.
- Ying QL, Nichols J, Evans EP, et al. Changing potency by spontaneous fusion. Nature 416 (2002): 545–8.
- Pells S, Di Domenico AI, Gallagher EJ, et al. Multipotentiality of neuronal cells after spontaneous fusion with embryonic stem cells and nuclear reprogramming in vitro. Cloning Stem Cells 4 (2002): 331–8.
- Chakraborty AK; Sodi S; Rachkovsky M, et al. A Spontaneous Murine Melanoma Lung Metastasis Comprised of Host × Tumor Hybrids. Cancer Res 60 (2000): 2512-2519.
- Chakraborty AK, Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant 34 (2004): 183-186.
- Pawelek J, and Chakraborty AK. Fusion of primary tumor cells with migratory bone marrow-derived cells provides a unifying explanation of metastasis. Nature Reviews Cancer 8 (2008): 377-386.
- Cowan CA, Atienza J, Melton DA, et al. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309 (2005): 1369–1373.
- Yu J, Vodyanik MA, He P, et al. Human embryonic stem cells reprogram myeloid precursors following cell-cell fusion. Stem Cells 24 (2006): 168–176.
- Marks JW. DEFINITION OF CELL FUSION. Reviewed on (2021).
- Rosenkrantz JL, Martinez M, Adithi Mahankali A, et al. Investigation of Human Endogenous Retrovirus-K (ERVK) Expression and Function in Normal Placentation and Preterm Pregnancy bioRxiv (2021).
- Aboul-Soud MAM, Alzahrani AJ, Mahmoud A. 2021. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 10 (2021): 2319.
- Segev N, Avinoam O, and Podbilewicz B. 2018. Fusogens. Current Biology 28 (2018): R367–R420.
- Ambrosi DJ, Rasmussen TP. Reprogramming mediated by stem cell fusion. J. Cell. Mol Med 9 (2005): 320-330.
- Matveeva NM, Shilov AG, Kaftanovskaya EM, et al. In vitro and in vivo study of pluripotency in intraspecific hybrid cells obtained by fusion of murine embryonic stem cells with splenocytes. Mol. Reprod. Dev 50 (1998): 128–138.
- Tada M, Takahama Y, Abe K, et al. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol 11 (2001): 1553–8.
- Hatano SY, Tada M, Kimura H, et. al. 2005. Pluripotential competence of cells associated with Nanog activity. Mech Dev 122 (2005): 67–79.
- Brown KL, Fisher AG, Reprogramming lineage identity through cell–cell fusion. Current Opinion in Genetics & Development 70 (2021): 15-23.
- Gurdon J. The developmental capacity of nuclei taken from intestinal epithelial cells of feeding tadpoles. J Embryol Exp Morphol 10 (1962): 622-640.
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev 24 (2010): 2239-2263.
- Zito F, Lampiasi N, Kireev I, et al. United we stand: adhesion and molecular mechanisms driving cell fusion across species. Eur J Cell Biol 95 (2016): 552-562.
- Harris H, Watkins JF. Hybrid cells derived from mouse and man: artificial heterokaryons of mammalian cells from different species. Nature 205 (1965): 640-646.
- Miller RA, Ruddle FH. Pluripotent tetracarcinoma-thymus somatic cell hybrids. Cell 9 (1976): 45-55.
- Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 99 (1987): 371–82.
- Hajkova P, Erhardt S, Lane N, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev 117 (2002): 15–23.
- Flasza M, Shering AF, Smith K, et al. Reprogramming in inter-species embryonal carcinoma-somatic cell hybrids induces expression of pluripotency and differentiation markers. Cloning Stem Cells 5 (2003): 339–54.
- Hasegawa K, Zhang P, Wei Z et al. Comparison of Reprogramming Efficiency Between Transduction of Reprogramming Factors, Cell–Cell Fusion, and Cytoplast Fusion, Stem Cells 28 (2010): 1338–1348.
- Yamanaka S, Blau HM. 2010. Nuclear reprogramming to a pluripotent state by three approaches. Nature 465 (2010): 704-12.
- Appleby SJ, Oback FC, Dhali A, et al. Double cytoplast embryonic cloning improves in vitro but not in vivo development from mitotic pluripotent cells in cattle. Front Genet 13 (2022): 933534.
- Park I-H, Lerou P-H, Zhao R, et al. Generation of human-induced pluripotent stem cells. Nat Protoc 3 (2008): 1180–1186.
- Matoba S, Zhang Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell 23 (2018): 471-485.
- Aigner B, Kessler B, Klymiuk N, et al. Genetically Tailored Pig Models for Translational Biomedical Research, Editor(s): P. Michael Conn, Animal Models for the Study of Human Disease (Second Edition), Academic Press 26 (2017): 671-701.
- Gouveia C, Huyser C, Egli D, et al. Lessons Learned from Somatic Cell Nuclear Transfer. Int J Mol Sci 21 (2020): 2314.
- Polejaeva IA. 25th ANNIVERSARY OF CLONING BY SOMATIC CELL NUCLEAR TRANSFER Generation of genetically engineered livestock using somatic cell nuclear transfer. Reproduction 162 (2021): F11–F22.
- Al Abbar A, Ngai SC, Nograles N, et al. Induced Pluripotent Stem Cells: Reprogramming Platforms and Applications in Cell Replacement Therapy. Biores Open Access 9 (2020): 121-136.
- Omole AE, Fakoya AOJ. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. Peer J 6 (2018): e4370.
- Do JT, and Scholer HR. Comparison of neurosphere cells with cumulus cells after fusion with embryonic stem cells: reprogramming potential. Reprod. Fertil. Dev 17 (2005): 143–149.
- Sumer H, Jones KL, Liu J, et al. Transcriptional changes in somatic cells recovered from embryonic stem– somatic heterokaryons. Stem Cells Dev 18 (2009): 1361–1368.
- Wong CC, Gaspar-Maia A, Ramalho-Santos M, et al. High efficiency stem cell fusion-mediated assay reveals Sall4 as an enhancer of reprogramming. PLoS ONE 3 (2008): e1955.
- Chakraborty AK, Pawelek J, Ikeda Y, et al. Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyl transferase V,1-6 branching, and metastasis. Cell Growth and Differ 12 (2001): 623-630.
- Rachkovsky M, Sodi S, Chakraborty AK, et al. Melanoma/ macrophage fusion hybrids with enhanced metastatic potential. Exp. Metastasis 16 (1998): 299-312.
- Sodi SA, Chakraborty AK, Platt JT, et al. Melanoma x macrophage fusion hybrids acquire increased melanogenesis and metastatic potential: Altered N-glycosylation as an underlying mechanism. Pigment Cell Res 11 (1998): 299-309.
- Chakraborty AK, de Freitas Sousa J, Espreafico EM, et al. Human monocyte x mouse melanoma fusion hybrids express human gene. GENE 275 (2001): 103-106.
- Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416 (2002): 542–5.
- Gage FH. 2000. Mammalian neural stem cells. Science 287 (2000): 1433-8.
- Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 115 (2005): 102–9.
- Redmond DE Jr, Bjugstad KB, Teng YD, et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA 104 (2007): 12175–80.
- Chen W, Huang Q, Ma S, et al. Progress in Dopaminergic Cell Replacement and Regenerative Strategies for Parkinson's Disease. ACS Chem Neurosci 10 (2019): 839-851.
- Playne R, Connor B. Understanding Parkinson's Disease through the Use of Cell Reprogramming. Stem Cell Rev Rep 13 (2017): 151-169.
- Kim J, Efe JA, Zhu S, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA 108 (2011): 7838–7843.
- Zhu S, Ambasudhan R, Sun W, et al. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res 24 (2014): 126–129.
- Zhang Y, Cao N, Huang Y, et al. Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 18 (2016): 368–381.
- Sheng C, Jungverdorben J, Wiethoff H, et al. A stably self-renewing adult blood-derived induced neural stem cell exhibiting patternability and epigenetic rejuvenation. Nat Commun 9 (2018): 4047.
- Choi DH, Kim JH, Kim SM, et. al. 2017. Therapeutic potential of induced neural stem cells for Parkinson's disease. Int J Mol Sci 18 (2017): 224-41.
- Wu J, Sheng C, Liu Z, et al. 2015. Lmx1a enhances the effect of iNSCs in a Parkinson's disease model. Stem Cell Res 14 (2015): 1–9.
- Xiao D, Liu X, Zhang M, et al. Direct reprogramming of fibroblasts into neural stem cells by single non-neural progenitor transcription factor Ptf1a. Nat Commun 9 (2018): 2865-2884.
- Hallett PJ, Deleidi M, Astradsson A, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 16 (2015): 269–274.
- Kim J, Su SC, Wang H, et al. 2011. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 9 (2011): 413–419.
- Chakraborty A, Diwan A. Similarities among Alzheimer’s Disease, Parkinson’s Disease and Dementia May Call for a Similar Treatment. Neurol Curr Res 2 (2022): 1014-1020.
- Ellison JM. Alzheimer’s and Parkinson’s Disease: Similarities and Differences Bright Focus Foundation. Swank Center for Memory Care and Geriatric Consultation, Christiana Care. Expert Advice. Published on: July 5 (2021).
- Chen Y, Liu Q, Liu J, et al. Revealing the Modular Similarities and Differences Among Alzheimer's Disease, Vascular Dementia, and Parkinson's Disease in Genomic Networks. Neuromolecular Med 24 (2022): 125-138.
- Kelly J, Moyeed R, Carroll C, et al. Genetic networks in Parkinson's and Alzheimer's disease. Aging (Albany NY) 12 (2020): 5221-5243.
- Rana P, Franco EF, Rao Y, et al. Evaluation of the Common Molecular Basis in Alzheimer's and Parkinson's Diseases Int J Mol Sci 20 (2019): 3730.
- Ruffini N, Klingenberg S, Schweiger S, et al. Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale. Cells 9 (2020): 2642.
- Sadlon A, Takousis P, Alexopoulos P, et al. miRNAs Identify Shared Pathways in Alzheimer's and Parkinson's Diseases. Trends Mol Med 25 (2019): 662-672.
- Dinda B, Dinda M, Kulsi G, et al. Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review. Eur J Med Chem 169 (2019): 185-199.
- Blurton-Jones M, Kitazawaa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 106 (2009): 13594-9.
- Boller F, Mizutani T, Roessmann U, et al. Parkinson disease, dementia, and Alzheimer disease: Clinicopathological correlations. Ann Neurol 7 (1980): 329-35.
- Kordower JH, Winn SR, Liu YT, et al. The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cell secreting human nerve growth factor. Proc Natl Acad Sci. USA 91 (1994): 10898–902.
- Zhang Q, Wu H-H, Wang Y, et al. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J Neurochem 136 (2015): 815–82.
- Lee IS, Jung K, Kim IS, et al. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol Neurodegener 10 (2015): 38-54.
- Lilja AM, Malmsten L, Röjdner J,Voytenko L, et al. Neural stem cell transplant-induced effect on neurogenesis and cognition in Alzheimer Tg2576 mice is inhibited by concomitant treatment with amyloid-lowering or cholinergic 7 nicotinic receptor drugs. Neural Plast (2015): 370432.
- Ager RR, Davis JL, Agazaryan A, et al. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus. 25 (2015): 813–26.
- Koch G, Motta C, Bonnì S, et al. Effect of Rotigotine vs Placebo on Cognitive Functions Among Patients with Mild to Moderate Alzheimer DiseaseA Randomized Clinical Trial. JAMA Netw Open 3 (2020): e2010372.
- Meiser J, Weindl D, Hiller K. 2013. Complexity of dopamine metabolism. Cell Commun Signal 11 (2013): 34.
- Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line- derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J. Neurosci 21 (2001): 581-589.
- Boyd JG and Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically naxotomizedmotoneurons in vivo. Exp. Neurol 183 (2003): 610-9.
- Chakraborty A and Diwan A. Autocrine and paracrine stimulation of dopamine secretion by human neural stem cells: Role BDNF and GDNF. Neurol Neurosci 3 (2020): 1-6.
- Moghadam FH, Alaie H, Karbalaie K. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation 78 (2009): 59–68.
- Xuan AG, Luo M, Ji WD. Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci Lett 45092 (2009): 167–71.
- Chen C, Xiao SF. Induced pluripotent stem cells and neurodegenerative diseases. Neurosci Bull 27 (2011): 107–114.
- Wu S, Sasaki A, Yoshimoto R, et al. Neural stem cells improve learning and memory in rats with Alzheimer's disease. Pathobiology 75 (2008): 186–194.
- Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology 33 (2013): 491–504.
- Mucke L. Neuroscience: Alzheimer's disease. Nature. 461 (2009): 895-7.
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508 (2011): 1-12.
- German CL, Baladi MG, McFadden LM. Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol Rev 67 (2015): 1005-24.
- Franz-Josef M, Evan S, Jeanne L. 2006. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci 7 (2006): 75-84.
- Kim JS, Choi HW, Choi S, et al. Reprogrammed pluripotent stem cells from somatic cells. Int J Stem Cells. 4 (2011): 1-8.
- Takahashi J. Strategies for Bringing Stem Cell-Derived Dopamine Neurons to the Clinic: The Kyoto trial. Prog Brain Res 230 (2017): 213-226.
- Westphalen D and Ghoshal M. Understanding Dopamine Agonists. Healthline, December 6 (2019).
- Holmqvist S, Lehtonen S, Chumarina M, et al. Creation of a library of induced pluripotent stem cells from parkinsonian patients. NPJ Parkinson’s Dis 2 (2016): 16009–16009.
- Ebrahimi A, Keske E, Mehdipour A, et. al. Somatic cell reprogramming as a tool for neurodegenerative diseases. Biomedicine & Pharmacotherapy 112 (2019): 108663.
- Vannucci L, Lai M, Chiuppesi F, et al. Viral vectors: a look back and ahead on gene transfer technology. Gene 36 (2013): 1–22.
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302 (2003): 415-9.
- Cong L, Ann Ran F, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 339 (2013): 819–23.
- Seki A, Rutz S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T. Cell 215 (2018): 985–97.
- Grath A, Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J Biol Eng. 13 (2019): 14.
- Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol Ther 29 (2021): 464-488.
- Kalra RS, Dhanjal JK, Das M, et al. Cell Trans differentiation and Reprogramming in Disease Modeling: Insights into the Neuronal and Cardiac Disease Models and Current Translational Strategies. Cells 10 (2021): 2558.
- Zhang J, Chu LF, Hou Z, et al. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc Natl Acad Sci. USA 114 (2017): E6072–E6078.
- Engler AJ, Sen S, Sweeney HL, et al. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126 (2006): 677–89.
- Miroshnikova YA, Nava MM, Wickstro SA. Emerging roles of mechanical forces in chromatin regulation. J Cell Sci 130 (2017): 2243–50.


 Impact Factor: * 5.814
Impact Factor: * 5.814 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 