STRA6: The key to inflammatory pathways in COVID-19
Article Information
Aziz Rodan Sarohan
Private Güney Hospital, Department of Obstetrics and Gynecology, Istanbul, Turkey
Shagreen Health Life Science. Be?tepe Mah. Nergiz sok. No:7A/9 Yenimahalle/ ANKARA/Turkey
*Corresponding author: Aziz Rodan Sarohan, Private Güney Hospital, Department of Obstetrics and Gynecology, Istanbul, Turkey
Received: 19 January 2023; Accepted: 27 January 2023; Published: 16 February 2023
Citation: Aziz Rodan Sarohan. STRA6: The key to inflammatory pathways in COVID-19. Fortune Journal of Health Sciences 6 (2023): 45-53.
View / Download Pdf Share at FacebookAbstract
The COVID-19 pandemic, caused by SARS-CoV-2, has infected more than 260 million people worldwide, causing more than 5.2 million deaths. Unlike other viral infections, COVID-19 is characterized by widespread and severe systemic effects, immune dysregulation, pneumonia, ARDS, and multiple organ damage. It also causes serious inflammatory, autoimmune, endocrine, and neuropsychiatric diseases also called post-COVID syndromes. This broad spectrum of disease seen in COVID-19 cannot be explained by the previously described mechanism of viral tropism based on a single receptor mediated by ACE2 and TMPRSS2 receptors. The pathogenesis of COVID-19 can only be explained by the influence of many receptors and signaling pathways. At a crossroads in retinol metabolism and multiple inflammation-related cellular signaling pathways, STRA6 plays a key role in the pathogenesis of COVID-19. Systemic organ involvement, neuroendocrine involvement, and immunological involvement in COVID-19 can be explained very clearly through STRA6 signaling. Retinoid metabolism and STRA6 activity interact. It has been previously shown that retinol levels are reduced in COVID-19. Due to retinol depletion in COVID-19, the functions of STRA6 are also impaired. This leads to the disruption of multiple cellular signaling pathways associated with inflammation. STRA6 has a key role in the regulation of inflammatory pathways and retinoid signaling in the pathogenesis of COVID-19 and is at the center of the inflammatory process in COVID-19 due to the multiple cellular signaling pathways it directs. Targeting STRA6 and its associated signaling pathways may yield results in the prevention and treatment of many inflammatory diseases as well as COVID-19.
Keywords
COVID-19, SARS-CoV-2, cytokine storm, inflammation, Treg, Th17, immune dysregulation, COVID-19 pathogenesis, viral tropism, spike protein, ACE2, STRA6, GPCR, omicron, multi-receptor mechanism, retinol, retinoid signaling, IT index
COVID-19 articles, SARS-CoV-2 articles, cytokine storm articles, inflammation articles, Treg articles, Th17 articles, immune dysregulation articles, COVID-19 pathogenesis articles, viral tropism articles, spike protein articles, ACE2 articles, STRA6 articles, GPCR articles, omicron articles, multi-receptor mechanism articles, retinol articles, retinoid signaling articles, IT index articles
Article Details
1. Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), infected more than 260 million people worldwide, causing more than 5.2 million deaths [1]. It seems that some success has been achieved against the epidemic with vaccines and strict isolation measures developed to combat the COVID-19 epidemic with an extraordinary effort around the world [2, 3]. The emergence of new SARS-CoV-2 mutants and recombinant strains has unfortunately reduced the effectiveness of vaccines in the fight against the pandemic [3]. For this reason, it is returned to the beginning every 3-4 months in the epidemic. Despite the vaccine, the number of patients who had severe COVID-19 and died is not small [4]. Therefore, it remains important to clarify the pathogenesis of COVID-19 clearly. Developing effective vaccines and drugs against COVID-19 will only be possible with a very good understanding of pathogenesis. Omicron, the most recently emerged variant of SARS-CoV-2, started to slow down the pandemic due to the fact that it did not cause severe disease despite its rapid transmission [2, 3]. With this development, the devastating effect of COVID-19 on societies has begun to diminish. The pathogenesis of COVID-19 should be well understood in order to combat similar epidemics that may occur in the coming years and to manage many chronic autoimmune, inflammatory, degenerative, and neuropsychiatric diseases that emerged after COVID-19 and are now called diseases.
The wide spectrum of diseases seen in COVID-19 cannot be explained by the viral tropism mechanism mediated by ACE2 and TMPRSS2 receptors [5]. The systemic effects and post-COVID syndromes in COVID-19 cannot be explained by the mechanism of viral tropism mediated by ACE2 receptors. These effects in COVID-19 can be explained by the multi-receptor mechanism and retinoid signaling disorder involving many receptors and many signaling pathways [5]. STRA6, one of the receptors and signaling pathways involved in the pathogenesis of COVID-19, has a very important role due to retinol metabolism and the inflammatory signaling pathways it directs. Therefore, STRA6 has a key role in the pathogenesis of COVID-19 [5, 6]. Likewise, the pathogenesis mechanism in COVID-19 can be clearly explained by retinoid signaling disorder and STRA6-directed signaling pathways. STRA6 and its associated signaling pathways play important role in this COVID-19 pathogenesis mechanism [5, 6].
Retinoid signaling disorder occurs due to retinol depletion (vitamin A deficiency) and metabolic defects occurring in COVID-19. The findings and symptoms here are common with many other inflammatory diseases, and it should not be overlooked that vitamin A deficiency is the etiology of many of them [7, 8, 9, 10]. Vitamin A is a powerful anti-inflammatory agent and its deficiency has been known for years to be associated with the inflammatory process [8, 9]. Some previous studies have shown that retinol levels decrease in severe COVID-19 patients and that retinol can be completely depleted in severe disease [11, 12]. STRA6 and retinoid activity interact with each other. Decreased STRA6 activity, which is synthesized due to retinoid signaling activity, impairs the retinoid signaling mechanism [6]. Since the disruption of STRA6 function affects many cellular signaling pathways, it causes the inflammatory process to go out of control, leading to pathogenetic consequences [6]. Therefore, the STRA6 receptor has a key role in the pathogenesis of COVID-19, in the regulation of retinoid metabolism, and in the control of inflammatory pathways. We think that STRA6 and the retinoid signaling pathway represent a target receptor and signaling pathway for the prevention and treatment of many inflammatory diseases as well as COVID-19.
2. STRA6 key to inflammatory pathways, retinoid signaling disorder, and COVID-19
Retinoids are a group of chemicals related to vitamin A that are essential for chordate mammals. It has been involved in many basic biological processes, including embryogenesis and vision. Retinol is transported to target tissues by binding to the retinol-binding protein (RBP) in the bloodstream and taken into the cell by binding to STRA6 [13,14]. Intracellular retinol is involved in genomic and nongenomic biological activities after conversion to retinoic acids (RA). A two-step dehydrogenase-catalyzed enzymatic reaction is required for the production of RA from retinol. Retinol is first converted to retinal and then irreversibly converted to RAs. RAs interact with a nuclear retinoic acid receptor (RAR) and retinoid X receptor (RXR) to regulate the expression of targeted genes [15, 16]. Retinoid levels are tightly controlled from intake to metabolism and subsequent cellular effects to prevent toxicity. Retinoic acid metabolism is mainly provided through the CYP450 (A26, B26, and C26 subgroups) monooxidase system.
Transmembrane transporter and signaling receptor STRA6, which is one of the important components of the retinoid metabolism network, plays a critical role in facilitating the cellular entry and exit of retinol [13]. STRA6 stimulated by endogenous RA levels and CRBP-1 supports retinol uptake into the cell [6]. The maintenance of normal cellular functions depends on vitamin A homeostasis and normal levels of endogenous retinoic acids [7, 13, 14]. Plasma retinol binding protein (RBP) is a specific transporter in plasma of retinol, the most common form of vitamin A. The integral membrane receptor STRA6 initiates cellular uptake of vitamin A by recognizing RBP-retinol and triggering the release and internalization of retinol [14]. The STRA6 transmembrane protein is the first protein to be recognized as a cytokine signal carrier, in addition to working as a cell surface receptor that facilitates cellular uptake of retinol by binding to the retinol-binding protein [13].
STRA6 initiates cellular retinol uptake in immune cells to improve immune system homeostasis in diverse populations [17]. Due to the critical function of vitamin A in the development of immune cells, STRA6 can be expressed in varying amounts in all subseries of peripheral blood mononuclear cells [17]. In a recent study, all T cells, monocytes, natural killer cells, and dendritic cell subsets were found to express the retinol-binding receptor STRA6 [17]. Many independent studies have found that STRA6 mediates the uptake of retinol from RBP into the cell and carries out this function together with intracellular proteins such as CRABP-1 [15, 16]. STRA6 plays a critical role in regulating cellular retinoic acid levels [17, 18]. Retinoic acids are also an important morphogen [15]. Retinol and retinoic acid metabolism and regulation are critical in the process of organogenesis. It has been determined that both vitamin A deficiency and excess vitamin A can have a teratogenic effect [19]. Likewise, STRA6 mutations, which are key in retinol regulation, are known to cause a wide range of complications related to malformations such as congenital heart defects, lung hypoplasia, anophthalmia, alveolar capillary dysplasia, diaphragmatic hernia, and mental retardation [20]. Recent findings show that mutations in the STRA6 gene are associated with congenital eye malformations, coloboma, and anophthalmia [21]. Single nucleotide polymorphisms or mutations (SNPs) in STRA6 are associated with the recurrence of type 2 diabetes [22]. In addition, Pasutto et al. showed that STRA6 mutations are responsible for many lung malformations, diaphragm anomalies, eye and heart defects, and mental retardation in humans, as in Matthew-Wood syndrome [23]. Although it is not as severe as in individuals with mutant STRA6, the STRA6 null mutation caused a significant reduction of retinoid in the neurosensory retina and retinal pigment epithelium and decreased eye morphology and visual responses [24].
STRA6 can act not only as a receptor of the vitamin A transporter but also as a cytokine receptor. After binding to Holo-RBP, STRA6 is directly phosphorylated at the tyrosine residue 643 site, which triggers and recruits STAT5 and Janus Kinase 2 gene (JAK2) activation [25]. Recent evidence suggests that STRA6 may function not only as a retinoid transporter but also as a switch on its own, a complex signaling center [26, 27, 28]. STRA6 determines cell fate by integrating retinoid signaling with many different signaling pathways such as p53, JAK/STAT, Wnt/catenin, SOCS, and calcium calmodulin [13, 28, 29, 33]. STRA6 is synthesized and functions by a healthy metabolism of retinol and retinoic acid. In this context, impaired retinol and retinoid metabolism in COVID-19 cause disruptions in the synthesis and functions of STRA6. The inevitable consequence of this is loss of control of STRA6 over inflammatory signaling pathways and excessive inflammatory cytokine release.
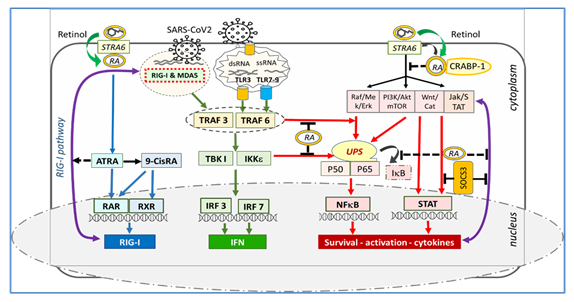
In Figure 1, STRA6 appears to be at the crucial junction of retinol's receptor, transporter, metabolic regulator, and cellular signaling. While inflammatory cascades are kept within physiological limits by the rhythmic activity of STRA6, tetanic stimulation or downregulation of STRA6 results in an excessive inflammatory process [6]. The MAPK, PI3K/Akt and Jak/STAT p53, Wnt/catenin, SOCS, and calcium calmodulin pathways are under the control of the non-genomic signaling of retinoic acids. Here, non-genomic signaling cascades are modulated by STRA6 and membrane-associated RARs through RA recruitment, leading to activation or inactivation of NFkB [6, 29, 30]. It has been found that SOCS and STAT3 signaling pathways are disrupted in COVID-19 and are associated with severe clinical pictures [31, 32]. Since the genes responsible for STRA6 synthesis are retinol-dependent genes [33], the decrease in endogenous retinoic acid levels due to retinol depletion may cause loss of synthesis and function of the STRA6 receptor. Retinol depletion in COVID-19 leads to both down-regulation and blockade (down-regulation) of the STRA6 receptor, leading to retinoid signaling disorder and excessive inflammatory process [6, 31, 32, 33]. Figure 1.
Figure 1: Association of STRA6 with retinol, retinoic acids, and CRABP-1. STRA6 inhibits NFkB activation and inflammatory cytokine release by inhibiting MAPK, PI3K/Akt, and Jak/STAT tonic signaling. RA depletion and downregulation of STRA6 cause decompression of MAPK, PI3K/Akt, JAK/STAT, Wnt/catenin, and calcium calmodulin pathways, NFkB, and STAT activation, and excessive inflammatory cytokine discharge.
3. STRA6 and retinoid signaling disorders explains neuroendocrine disorders in COVID-19
The association of STRA6 with retinoid signaling and cholesterol and steroid hormone regulation may also explain the neuroendocrine disorders very common in COVID-19 patients. Diabetes, thyroid, reproductive and adrenal disorders are very common in COVID-19. Endocrine problems in COVID-19 have an important place in both acute disease and chronic residual diseases defined as post-COVID syndromes [34]. In addition to glucose regulation disorder, severe deterioration in cholesterol regulation occurs in COVID-19 [36, 37]. STRA6 mediates the uptake of cholesterol as well as retinol [35, 39]. It has been found that plasma cholesterol levels decrease in COVID-19 and this is associated with severe disease [36, 37].
A sufficient number of studies have accumulated in the literature regarding the role of cholesterol regulation in COVID-19, but sufficient explanation has not been made about the mechanism by which cholesterol is involved in the pathogenesis of COVID-19 and its causes and consequences [37]. As is known, cholesterol is the precursor of steroid hormones and has been associated with severe disease in COVID-19 patients [38, 39]. In COVID19, it has been shown in many studies that cholesterol decreases as well as retinol in patients with COVID-19 [11, 12, 36, 37, 38]. Retinoic acids and STRA6 are involved in the transport and regulation of cholesterol into cells. Especially in immune system cells, retinoic acids upregulate ABCA1 expression and provide cholesterol flow [35, 39]. We think that the dysfunction of STRA6 and the development of retinoid signaling defect in COVID-19 may cause serious disruption in steroid hormone synthesis and structural and functional disorders in many endocrine organs since cholesterol is a precursor in steroid hormone synthesis.
Serious anatomical and functional disorders have been reported in the adrenal glands, thyroid, ovary, and testes, especially the hypothalamus, which is the neuroendocrine regulation center, in COVID-19 [40, 43, 44]. In addition to diabetes and hypothyroidism, menstrual and reproductive disorders due to low estrogen and testosterone, and adrenal dysfunction due to adrenal gland damage have also been reported very frequently in COVID-19 patients [41, 42, 43]. It has been reported that testosterone and estradiol levels decrease in COVID-19 [44, 45]. In addition to being a disease that predisposes to severe COVID-19, diabetes can occur due to COVID-19 due to anatomical and metabolic disorders on the pancreas and peripheral tissues of COVID-19 [42]. We think that the adrenal gland is affected in two different ways and in two stages in COVID-19. In the first stage, which is the early stage of the disease, the hypothalamic-pituitary-adrenal axis gains excessive activity due to the stress brought on by the disease, causing cortisol levels to rise. This is a pathological hormonal response that develops with emotional effects and the neuroadrenal center being affected. However, due to the worsening of the disease and excessive inflammatory mediator release, ischemic damage and adrenal insufficiency develop in the adrenal gland. At this stage, serum cortisol levels are found to be low in patients. There are also studies reporting both high and low serum cortisol levels in COVID-19 patients [46, 47].
Dysfunction and retinoid signal defect in STRA6 are responsible for both acute systemic effects and chronic post-COVID neuroendocrine syndromes in COVID-19 [5, 6]. Neurological disorders seen in COVID-19, cardiovascular disorders, hormonal imbalances, immune system dysregulation, excessive inflammatory response (cytokine storm), lymphopenia, ineffective RIG-I activity, Type-I interferon synthesis disorder, thrombosis tendency, loss of smell and taste, and STRA6 dysfunction results from retinol depletion and retinoid signaling impairment [6, 7]. It should not be overlooked that the tissues most active in terms of retinoid metabolism are the parts most affected in COVID-19 [48, 49]. The correlation between COVID-19 and retinoid activity reveals the cause-effect relationship between COVID-19 and retinoid metabolism [50].
Systemic organ involvement, brain involvement, and endocrine organ involvement in COVID-19 cannot be explained by the viral tropism mechanism explained by ACE2 [5]. Likewise, ACE2 receptors, which enable the internalization of SARS-CoV-2 into host cells, are either absent or poorly expressed in these severely affected organs. Likewise, it is known that STRA6 is very intensely expressed in these tissues [54, 55, 56]. Virus could not be isolated in damaged tissues, especially testicular and adrenal tissues of some patients severely affected by COVID-19 [47, 57, 58]. Postmortem adrenal tissue examination of COVID-19 patients revealed infarction, thrombus, hemorrhage and necrosis in the adrenal tissue, but SARS-CoV- 2 genomes could not be detected [47]. All this shows us that the virus causes retinoid signaling disorder rather than direct damage, causing systemic effects and severe inflammation and multi-organ damage.
In addition, retinoic acids have assumed critical physiological roles such as synaptic plasticity, learning and memory, adult neurogenesis, and hormone production [30, 48]. Retinoic acid deficiency causes progenitor cell differentiation failure in the olfactory epithelium, and as a result, olfactory neuron differentiation does not occur [48]. An explant system has shown that if retinoic acid synthesis fails, retinoic acid synthesis in the olfactory epithelium is inhibited, resulting in olfactory dysfunction [48]. Extensive neuropsychiatric and endocrine problems in COVID-19 [49, 50] can be explained by the deterioration of the effectiveness of retinoid signaling in sensory and nerve functions [50]. The olfactory dysfunction in COVID-19 is caused by the cessation of differentiation of progenitor cells in the olfactory epithelium to sensory neurons due to retinol depletion and retinoid signaling defect, and the decrease in the synthesis and activity of the glutamine receptor GluR1, which is responsible for the presynaptic transmission of the olfactory signal [6]. GluR1 is synthesized by the nongenomic activity of RAs [30].
4. STRA6, retinol depletion and biphasic immunopathogenesis in COVID-19
Disruption of retinol and cholesterol regulation provided by STRA6 leads to the impaired regulatory function of the immune system [51, 52, 53]. There are many studies on the effect of retinol on immune system regulation. Although cholesterol is not as much as retinol, it participates in the regulation of the immune system. Cholesterol levels also play important roles in immune cells such as monocyte priming, neutrophil activation, hematopoietic stem cell mobilization, and enhanced T cell production [59]. The cause of immune system dysregulation and biphasic immune pathogenesis in COVID-19, which is widely accepted by the scientific community, is retinol depletion and retinoid signaling disorder [6, 7]. Vitamin A and RAs have important modulatory functions in the immune system [51, 60]. In fact, retinol is an important hormone and immune system regulator. It works with zinc to improve the function of the immune system [7, 61].
Retinoids are retinyl esters, all-trans-retinal and all-trans retinoic acid (ATRA) [62], which have qualitative activity relative to retinol. RAs are involved in genomic and non-genomic pathways in the form of natural and adaptive responses via RA receptors (RAR/RXR) [13, 30, 63]. Retinoids are typically enhancers for NK cells, Innate Lymphoid Cells (ILCs), and antigen presenting cells (DCs). ATRA is required for the development of the immune system itself and for the development and homing of ILCs. The determination of cell fate of RAs, cell differentiation and wide range of effects on lymphocyte functioning are mediated by ATRA [52].
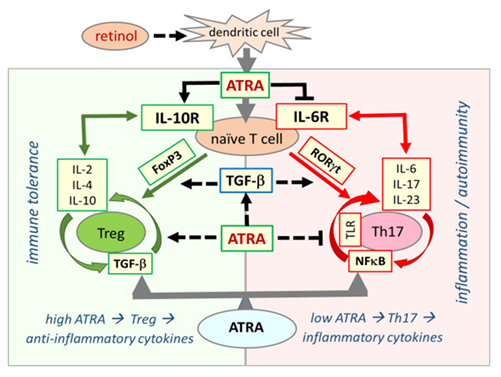
In the immune system, the effect of ATRA on the development of T and B lymphocytes has been shown in many studies [66, 67]. ATRA has a critical function in increasing regulatory T cells (Tregs) and regulating immune tolerance in the immune system [67]. When serum retinol is at normal concentrations, Treg dominance continues in immune system regulation. Treg dominance confers immune tolerance against the gut microbiota in the host while developing self-tolerance against its own tissues. In retinol depletion and retinoid signaling disorder, Treg dominance decreases, while effector Th17 cells, which cause excessive inflammatory cytokine release, take over. This causes inflammatory process activation, loss of self-tolerance, and immune tolerance. The main disorder underlying the pathogenesis of autoimmune diseases is immune dysregulation and Treg/Th17 cell imbalance [6, 7, 68]. Retinol depletion and impaired retinoid signaling mechanism underlie immune dysregulation, biphasic immune pathogenesis, and autoimmune syndromes in COVID-19. Figure2.
Figure 2: Retinoic acid signaling is essential in establishing immune tolerance through promoting differentiation of Tregs and inhibiting the development of proinflammatory Th17 cells. The balance between Treg and Th17 cells defines immune tolerance or inflammation. Both Treg and Th17 cells differentiate from naive T cells under different cellular and tissue milieu requiring TGF-β. In the presence of sufficient ATRA levels, the interaction between signaling pathways from RA, TGF-β, and cytokines blocks IL-6 signaling and induce FoxP3 transcription factor that ultimately leads to differentiation of naïve T cells into Tregs, which induce expression of anti-inflammatory cytokines, such as IL-2, IL-4, and IL-10, and suppress the inflammatory response that culminates in the establishment of self-tolerance (immune tolerance circle, green circled arrows). In the presence of low or no ATRA, retinoid signaling is weak or absent and fails to block IL-6 signaling, leading to the expression and activation of RORγt that reprogram differentiation of naïve T cells into pro-inflammatory Th17 cells. Effector Th17 cells have a high activity of NF-κB signaling that leads to the expression and release of various proinflammatory cytokines such as IL-6, IL-17, and IL-23, generating an inflammatory response that may lead to the development of autoimmune diseases. (inflammation circle, red circled arrows).
Impairment and dysfunction of STRA6 receptor synthesis due to retinol depletion and retinoid signaling disorder leads to disruption of retinol transfer to the cell, resulting in a decrease in endogenous retinoic acid levels and retinoid signaling disorder. Therefore, STRA6 and retinoid metabolism are interrelated and interdependent and intertwined processes. Retinoid signaling disorder causes immune system dysregulation, disruption of type I IFN synthesis, severe inflammatory cytokine release, and devastating systemic multi-organ damage in COVID-19 [6, 50]. Impaired retinoid signaling mechanism can also explain chronic, inflammatory, degenerative, autoimmune, neuroendocrine, and neuropsychiatric post-COVID syndromes [6, 50]. Impaired retinoid signaling mechanism also seems to be responsible for chronic, inflammatory, degenerative, autoimmune, neuroendocrine and neuropsychiatric post-COVID syndromes [6, 50].
Retinoids can directly stimulate the expression of Interferon-stimulated gene (ISG) Messenger RNA (mRNA) including IFN regulatory factor 1 (IRF-1) and retinoic acid-inducible gene I (RIG-I) [64]. RIG I signaling is essential in the production of antiviral Type I interferon and is responsible for the severe clinical picture resulting in interruption of type I IFN synthesis in COVID-19 due to retinol depletion. Type I IFN synthesis defects with excessive inflammatory reactions in COVID-19 are the main pathogenetic disorders [69]. ATRA can inhibit the release of the inflammatory cytokine IL6, which is central to the pathogenesis of COVID-19 [70]. Likewise, ATRA can also prevent lung fibrosis with its suppressor activity on IL6 [71].
It has been found in Vero E6 cells that ATRA can exert an antiviral effect by binding to SARS-CoV-2 3CLpro in cell culture and inhibit SARS-CoV-2 replication together with other retinoids [72]. Synthetic and natural retinoids also have potent inhibitory effects on the replication of many viruses such as MeV, cytomegalovirus, influenza, norovirus, and hepatitis B virus (HBV) [73, 74]. There is also additional evidence that retinoid signaling activation can effectively suppress coronaviruses. Japanese researchers have shown that SARS-CoV-2 replication can be inhibited by ATRA over RIG-I in cell culture [75].
5. Conclusion
Dysfunction of STRA6 due to retinol depletion in COVID-19 further deepens the problem of retinol depletion and retinoid signaling in COVID-19. Due to the interdependent nature of STRA6 and retinoid metabolism, a vicious circle occurs. STRA6 synthesis defect, which develops due to retinol depletion, causes a decrease in endogenous retinoic acid levels and a deepening of retinoid signaling disorder. As a result, multisystemic organ damage occurs due to immune dysregulation, suppression of the congenital immune system, lymphopenia, ineffective RIG-I pathway, type I interferon synthesis defect, and especially hyperactivation of the adaptive immune system and excessive inflammatory cytokine release (cytokine storm).
Retinoid signaling defect also causes acute COVID-19 findings such as neurological disorders, diabetes, hormonal imbalance, thrombosis and loss of smell and taste, and chronic, degenerative, inflammatory, autoimmune neuroendocrine, and neuropsychiatric syndromes referred to as post-COVID syndromes. In conclusion, we think that the STRA6 receptor plays a key role in the pathogenesis of COVID-19 due to its central location in cellular signaling pathways. Targeting STRA6 and the cellular signaling pathways it directs in COVID-19 may lead to the development of new treatments for the severe clinical picture of COVID-19 and post-COVID syndromes. In addition to the serious and systemic effects in COVID-19, it is obvious that inflammatory, degenerative, autoimmune, and neuropsychiatric syndromes are also associated with disruption of signaling via STRA6 and retinoid signaling disorder. In this respect, these studies for COVID-19 may open different doors for us to prevent and better manage these chronic diseases, which are not caused by microorganisms, but increase and spread in an epidemic.
Conflicts of interest
The author declares that there are no conflicts of interest regarding the content of this article.
Acknowledgements
I would like to thank Özlem ?entürk, for the English spelling corrections.
Funding
None
References
- World Health Organization-Official COVID-19 Information. Weekly epidemiological update on COVID-19-8 March (2022).
- When will the COVID-19 pandemic end? Sarun Charumilind, Matt Craven, Jessica Lamb, Adam Sabow, Shubham Singhal, and Matt Wilson.
- https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/when-will-the-covid-19-pandemic-end
- Natalia Teruel, Matthew Crown, Matthew Bashton, Rafael Najmanovich. Computational analysis of the effect of SARS-CoV-2 variant Omicron Spike protein mutations on dynamics, ACE2 binding and propensity for immune escape. Natalia Teruel, Matthew Crown, Matthew Bashton, Rafael Najmanovich. bioRxiv 12 (2021): 472622;
- Maren Schubert, Federico Bertoglio, Stephan Steinke, Philip Alexander Heine, Mario Alberto Ynga-Durand et.al. Human serum from SARS-CoV-2 vaccinated and COVID-19 patients shows reduced binding to the RBD of SARS-CoV-2 Omicron variant. medRxiv 2021.12.10.21267523; doi: https://doi.org/10.1101/2021.12.10.21267523 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497–506.
- Sarohan A R. Multi-receptor mechanism and retinoid signaling disorder in the pathogenesis of COVID-19 (2022).
- Sarohan AR, K?z?l M, ?nkaya AÇ, Mahmud S, Akram M, Cen O. A novel hypothesis for COVID-19 pathogenesis: Retinol depletion and retinoid signaling disorder. Cell Signal 87 (2021): 110121
- Sarohan AR. COVID-19: Endogenous Retinoic Acid Theory and Retinoic Acid Depletion Syndrome. Med Hypotheses 144 (2020): 110250.
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 21 (2001): 167-92
- Reifen R. Vitamin A as an anti-inflammatory agent. Proc Nutr Soc 61 (2002): 397-400
- Gieng SH, Green MH, Green JB, Rosales FJ. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J Lipid Res 48 (2007): 904-13.
- Aziz Rodan Sarohan, Hakan Akelma, E?ref Araç, Özgür Aslan. medRxiv (2021).
- Tepasse PR, Vollenberg R, Fobker M, Kabar I, Schmidt H, Meier JA, et al. Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients 13 (2021): 2173.
- Dhokia V, Macip S. A master of all trades – linking retinoids to different signalling pathways through the multi-purpose receptor STRA6. Cell Death Discov 7 (2021): 358.
- Chen Y, Clarke OB, Kim J, Stowe S, Kim YK, Assur Z, et al (). Structure of the STRA6 receptor for retinol uptake. Science (New York, N.Y.) 353 (2016): aad8266.
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Décimo D, Dollé P, et al. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 63 (1997): 173-86.
- Kawaguchi R, Yu J, Ter-Stepanian M, Zhong M, Cheng G, Yuan Q, et al. Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem Biol 10 (2011): 1041-51.
- Barrett, Crisha et al. “Human Peripheral Blood Mononuclear Cells Express High Levels of the Vitamin a Transport Protein, Stimulated by Retinoic Acid 6 (P19-004-19).” Current Developments in Nutrition 3 (2019): P19-004-19.
- Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci 53 (2012): 3027-39.
- Kin Ting Kam R, Deng Y, Chen Y. et al. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci 2 (2012): 11.
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. American Journal of Human Genetics 80 (2007): 550-60.
- Ng WY, Pasutto F, Bardakjian TM, Wilson MJ, Watson G, Schneider A, Mackey DA, Grigg JR, Zenker M, Jamieson RV. A puzzle over several decades: eye anomalies with FRAS1 and STRA6 mutations in the same family. Clin Genet 83 (2013): 162-8.
- Nair AK, Sugunan D, Kumar H, Anilkumar G. Case-control analysis of SNPs in GLUT4, RBP4 and STRA6: association of SNPs in STRA6 with type 2 diabetes in a South Indian population. PLoS One 5 (2010): e11444.
- Chassaing N, Golzio C, Odent S, Lequeux L, Vigouroux A, Martinovic-Bouriel J, et al. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum Mutat 30 (2009): E673-81
- Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. J Cell Metab 7 (2008): 258-68.
- Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. ProcNatlAcadSci U S A 108 (2011): 4340-5
- Berry DC, Croniger CM, Ghyselinck NB, Noy N. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol-binding protein receptor STRA6. Mol Cell Biol 32 (2012): 3851-9.
- Noy N. Signaling by retinol and its serum binding protein. Prostaglandins Leukot Essent Fatty Acids 93 (2015): 3-7.
- Berry DC, O'Byrne SM, Vreeland AC, Blaner WS, Noy N. "Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6". Molecular and Cellular Biology 32 (2012): 3164–75.
- Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol 19 (2008): 414-422.
- Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: Genomic and nongenomic effects. J Lipid Res 54 (2013): 1761–1775.
- Johnson HM, Lewin AS, Ahmed CM. SOCS, Intrinsic Virulence Factors, and Treatment of COVID-19. Front Immunol 11 (2020): 582102.
- Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. Hyperactivation of STAT3 occurs in COVID-19 infection and other viral infections. "An aberrant STAT pathway is central to COVID-19". Cell Death Differ 27 (2020): 3209–3225.
- Carrera S, Cuadrado-Castano S, Samuel J, Jones GD, Villar E, Lee SW, et al. Stra6, a retinoic acid-responsive gene, participates in p53-induced apoptosis after DNA damage. Cell Death Differ 20 (2013): 910-9.
- Bhadada SK. Managing common endocrine disorders amid COVID-19 pandemic. Diabetes Metab Syndr 14 (2020): 767-771.
- Liu Y, Zhong Y, Chen H, Wang D, Wang M, Ou JS, et al. Retinol-Binding Protein-Dependent Cholesterol Uptake Regulates Macrophage Foam Cell Formation and Promotes Atherosclerosis. Circulation 135 (2017): 1339-1354.
- Zhao M, Luo Z, He H, et al. Decreased Low-Density Lipoprotein Cholesterol Level Indicates Poor Prognosis of Severe and Critical COVID-19 Patients: A Retrospective, Single-Center Study. Front Med (Lausanne) 8 (2021): 585851.
- Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in Relation to COVID-19: Should We Care about It? J Clin Med 9 (2020): 1909.
- Zinellu A, Paliogiannis P, Fois AG, Solidoro P, Carru C, Mangoni AA. Cholesterol and Triglyceride Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front Public Health 9 (2021): 705916.
- Jiang H, Badralmaa Y, Yang J, Lempicki R, Hazen A, Natarajan V. Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux. Lipids Health Dis 11 (2012): 69.
- Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord 22 (2021): 803-815.
- Malik J, Zaidi SMJ, Waqar AU, Khawaja H, Malik A, Ishaq U, et al. Association of hypothyroidism with acute COVID-19: a systematic review. Expert Rev Endocrinol Metab 16 (2021): 251-257.
- Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: A bidirectional relationship. Clin Investig Arterioscler 33 (2021): 151-157.
- Sengupta P, Leisegang K, Agarwal A. The impact of COVID-19 on the male reproductive tract and fertility: A systematic review. Arab J Urol 19 (2021): 423-436.
- Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, et asl. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reproductive biomedicine online 42 (2021): 260–267.
- Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: A case-control study. Andrology 9 (2021): 1043-1052.
- Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf) 63 (2005): 197-202.
- Iuga AC, Marboe CC, M Yilmaz M, Lefkowitch JH, Gauran C, Lagana SM. Adrenal Vascular Changes in COVID-19 Autopsies. Arch Pathol Lab Med 144 (2020): 1159-1160.
- Bhaskar C, Pritam T, Radha K, Sasmita D, Sweta M, Ting C, et al. Retinoic Acid Signaling Pathways in Development and Diseases. Bio org Med Chem 22 (2014): 673–683.
- Maria G, Eleni K, Despina F, Ioannis NS, Efstathios K, Meletios A. Organ-specific manifestations of COVID-19 infectio. Clin Exp Med 20 (2020): 493–506.
- Sarohan AR. Systemic Organ Involvement and Retinoid Signaling Disorder in COVID-19. Immunogenet Open Access 6:145.
- Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci 1143 (2008): 170-87.
- Kim CH. Control of Innate and Adaptive Lymphocytes by the RAR-Retinoic Acid Axis. Immune Netw18 (2018): e1.
- Ross AC, Stephensen CB. Vitamin A and retinoids in antiviral responses. FASEB J 10 (1996): 979-85.
- Soni S, Jiang Y, Tesfaigzi Y, Hornick JL, Çataltepe S. Comparative analysis of ACE2 protein 54. expression in rodent, non-human primate, and human respiratory tract at baseline and after injury: A conundrum for COVID-19 pathogenesis. PLoS One 16 (2021): e0247510.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203 (2004): 631-7.
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 251 (2020): 228-248.
- Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril 113 (2020): 1135-1139.
- Flaifel A, Guzzetta M, Occidental M, Najari BB, Melamed J, Thomas KM, Deng FM. Testicular Changes Associated With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Arch Pathol Lab Med 145 (2021): 8-9.
- Aguilar-Ballester M, Herrero-Cervera A, Vinué Á, Martínez-Hervás S, González-Navarro H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients 12 (2020): 2021.
- Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of Vitamin A in the Immune System. J Clin Med 7 (2018): 258.
- Fortes C, Forastiere F, Agabiti N, Fano V, Pacifici R, Virgili F, et al. The effect of zinc and vitamin A supplementation on immune response in an older population. J Am Geriatr Soc 46 (1998): 19-26.
- Marill J, Idres N, Capron CC, Nguyen E, Chabot GG. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab 4 (2003): 1-10.
- Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol 66 (2006): 606–30.
- Wu SF, Xia L, Shi XD, Dai YJ, Zhang WN, Zhao JM, et al. RIG-I regulates myeloid differentiation by promoting TRIM25-mediated ISGylation. Proc Natl Acad Sci U S A 117 (2020): 14395-14404.
- Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic Acid. J Immunol 192 (2014): 2953-8.
- Marks E, Ortiz C, Pantazi E, et al. Retinoic Acid Signaling in B Cells Is Required for the Generation of an Effective T-Independent Immune Response. Front Immunol 7 (2016): 643.
- Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, et al. All-trans retinoic acid promotes TGF-β-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One 6 (2011): e24590.
- Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol 12 (2015): 553-557.
- Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369 (2020): 718-724.
- Zhang J, Hao Y, Ou W, et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med 18 (2020): 406.
- Tabata C, Tabata R, Nakano T. The calpain inhibitor calpeptin prevents bleomycin-induced pulmonary fibrosis in mice. Clin Exp Immunol 162 (2010): 560– 567.
- Morita T, Miyakawa K, Jeremiah SS, Yamaoka Y, Sada M, Kuniyoshi T, et al. All-Trans Retinoic Acid Exhibits Antiviral Effect against SARS-CoV-2 by Inhibiting 3CLpro Activity. Viruses 13 (2021): 1669.
- Isaacs CE, Kascsak R, Pullarkat RK, Xu W, Schneidman K. Inhibition of herpes simplex virus replication by retinoic acid. Antiviral Res 33 (1997): 117-27
- Baron S, Tyring SK, Fleischmann WR Jr, Coppenhaver DH, Niesel DW, Klimpel GR, et al. The interferons. Mechanisms of action and clinical applications. JAMA 266 (1991): 1375-83.
- Yamada T, Sato S, Sotoyama Y, Orba Y, Sawa H, Yamauchi H, et al. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat Immunol 22 (2021): 820-828.


 Impact Factor: * 5.814
Impact Factor: * 5.814 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 