Cost-effective pool Testing qRT-PCR Method for early Detection and mass Screening of SARS-CoV-2 Infection
Article Information
Samiksha Sharma#, Vaibhav K. Tamrakar#, Nishant Burnase, Shailendra S. Parihar, Kirti Pagarware and Rekha Barapatre*
Chhattisgarh Institute of Medical Sciences, Bilaspur, Chhattisgarh, India
#Equeal Contribution
*Corresponding author: Rekha Barapatre. Chhattisgarh Institute of Medical Sciences, Bilaspur, Chhattisgarh, India.
Received: 08 April 2023; Accepted: 17 April 2023; Published: 25 April 2023
Citation: Samiksha Sharma, Vaibhav K. Tamrakar, Nishant Burnase, Shailendra S. Parihar, Kirti Pagarware and Rekha Barapatre. Cost-effective pool Testing qRT-PCR Method for early Detection and mass Screening of SARS-CoV-2 Infection. Fortune Journal of Health Sciences. 6 (2023): 174-177.
View / Download Pdf Share at FacebookAbstract
The COVID-19 pandemic has affected millions of people all around the world. The molecular diagnostic method quantitative reverse transcriptase PCR (qRT-PCR) is considered the gold standard test for SARS-CoV-2 diagnostics. The current study focused on a pooled sample testing strategy and its evaluation in screening of SARS-CoV-2 clinical samples during a disease outbreak. In total, 1389 clinical samples were collected at the COVID-19 sample collection center, CIMS Bilaspur C.G. These samples were taken from the upper respiratory tract (Naso- and Oro-pharyngeal swabs) and kept in viral transport medium (VTM). Samples are randomly pooled in the desired cluster of samples during the lysis. All lysed samples are forwarded to extraction and then qRT-PCR is performed. Among 1389 samples, 600 were taken to make five pool simulations. Similarly, 789 samples were for three pool simulations and the results were compared to their individual test results. It has been found that both 3-sample and 5-sample pooling are almost equally accurate and showed a have a perfect agreement with individual sample testing. However, 3-sample pooling revealed low false negativity and had high concordance results with individual testing. Three pool simulations resulted in more précised and accurate diagnostic outcomes compared to 5 pool samples. Slightly false-negative results were seen in a few five pooled samples, which were further found positive upon retesting it in a single sample. It was observed that very minimal single positive samples with Ct values near 35-37 were likely to be missed in pooled sample analysis. This study concludes that the pool testing strategy should be considered an effective screening tool for mass sampling because this testing method also has less effect on the environment and reduces the cost of consumables.
Keywords
Cost-effective, COVID-19 pandemic, qRT-PCR, Pooling, SARS-CoV-2
Article Details
1. Introduction
The world faces the most crippling pandemic because of an unprecedented outbreak and global spread of COVID-19 after its first detection in Wuhan, China, in December 2019. COVID-19 is a substantial challenge for healthcare systems and their infrastructure and after its worldwide spread, it was declared a pandemic by WHO in March 2020 [1]. The most common symptoms displayed by individual infected with this virus includes fever, cough and sore throat. However, patients having diabetes, cardiovascular disease, chronic respiratory disease, or cancer become seriously ill and require immediate medical attention [2-4]. Testing helps in the detection of viruses in symptomatic as well as in asymptomatic individuals [5]. Many techniques have been used for diagnostic purposes, such as rapid antigen test, True-Nat, Chest CT-scan and qRT-PCR. To differentiate SARS-CoV-2 infection from other respiratory infections WHO has recommended more robust and sensitive tests. qRT-PCR-based diagnosis of COVID-19 is significant in confirming the infection and its containment. qRT-PCR is a susceptible technique as it can detect a single copy of the genome and multiply it into millions of copies resulting in qRT-PCR being considered the gold standard test for detecting SARS CoV-2 infection [6]. Due to its high sensitivity and specificity, qRT-PCR tests are also being performed as confirmatory tests for rapid antigen testing [7, 8]. Mass screening is needed to deal with this pandemic and overcome the load of clinical samples. The high demand for qRT-PCR tests led to a local shortage of resources, so to conserve resources and time, a pool testing strategy can be considered as a viable option. Many studies are available on qRT-PCR is also able to detect several variants of SARS CoV-2 [6]. Sample pooling has been shown as a cost-effective and efficient approach to conserve resources and time for testing a large number of samples [9, 10]. Present study aims to evaluate the feasibility of pool sample testing to enhance the mass screening potential of diagnostic laboratories.
2. Methodology
1389 samples were taken for this study from the upper respiratory tract (Naso- and Oro-pharyngeal swabs) and kept in a viral transport medium (VTM). All these samples have been collected at the COVID-19 sample collection center, CIMS Bilaspur C.G. All procedures were performed according to ICMR guidelines and regulations. Among 1389 samples, 600 were taken to make five pool simulations. Similarly, 789 samples for three pool simulations. For lysis, 140 µL sample was taken from three different VTM and pooled in one vial in case of 3 pool simulations. Then 140µL sample was taken from a pooled vial for RNA extraction. The same process was followed to make five pool simulations. The Viral RNA extraction was performed using the HiPurA viral RNA Purification kit (HIMEDIA, India) as per the manufacturer’s instructions. Once the RNA was extracted, 5μl was added to 20μl of the master mix (Real-Q 2019-nCoV Detection Kit BioSewoom, Korea). Positive control and negative control were used in each batch. qRT-PCR test was performed through BioRad CFX96 thermal cycler followed by thermal conditions 95°C for 15 min and then 40 cycles of 95°C for 15s, 62°C for 45s. Data analysis was done by using two-by-two tables. According to Cohen’s statistical law, the two-by-two table was prepared for three and 5-pool simulations against individual samples.
Cohen’s kappa is a statistical method commonly used to estimate the degree of agreement between two raters. Basically, it compares the probability of agreement between two independent variables.
The Cohen’s kappa degree of agreement between sample pooling tests and individual tests was performed by the following formula:
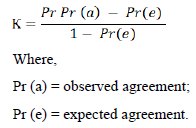
Cohen’s Kappa results could be interpreted as follows: values ≤ 0 indicates zero agreement, 0.01 – 0.20 represents none to the slight agreement, 0.21 – 0.40 as fair, 0.41–0.60 denotes moderate, 0.61–0.80 as substantial and 0.81–1.00 represents perfect agreement [11].
3. Result
Three pool and five pool simulations were prepared through 1389 swab samples. 128 pools of 600 specimens including five pool simulation were made, out of which 115 pools were found positive. After that, these pool simulations were tested individually, resulting in 128 positive samples. Individual testing was performed to obtain the number of truly positive samples. Sometimes, pool may found more than one positive sample. Cohen's kappa statistic for the 5-pool sample testing method was 0.932. Out of 600 samples, 13 samples were found discordant. The Ct value of these 13 samples is nearly 35-37. Similarly, 789 specimens were collected and merged into 263 pools to make 3-pool simulations. In this, 263 pools were reported as positive and while tested individually, all samples were found to have 100% concordance results. Cohen's kappa statistic for 3-Pool sample testing was 1.000. For the detection of COVID-19 infection in both, symptomatic and asymptomatic individuals 3-pool approach was found more suitable, sensitive and accurate than the 5-pool.[11] Positive and negative agreements between 3-pooled versus individual and 5-pooled versus individual testing were 100% and 93.8%, respectively (Table I and Table II).
Table I: Comparison of pool testing and individual testing of SARS-Cov-2 clinical specimen through qRT- PCR for 3-pool testing strategy
|
|
Individual qRT-PCR testing |
|||
|
Positive |
Negative |
Total |
||
|
Pool |
Positive |
263 |
0 |
263 |
|
Negative |
0 |
526 |
526 |
|
|
Total |
263 |
526 |
789 |
|
Table II: Comparison of pool testing and individual testing of SARS-Cov-2 clinical specimen through qRT-PCR for 5-pool testing strategy
|
|
Individual qRT-PCR testing |
|||
|
Positive |
Negative |
Total |
||
|
Pool |
Positive |
115 |
0 |
115 |
|
Negative |
13 |
472 |
485 |
|
|
Total |
128 |
472 |
600 |
|
4. Discussion
During the COVID-19 pandemic, the deficit of laboratory consumables, lack of trained human resources and shortage of kits for RNA extraction as well as for qRT-PCR has become an issue of concern [12, 13]. A pooling strategy could be used to overcome all these issues. This study evaluates the cost-effectiveness and diagnostic sensitivity of sample pooling through qRT-PCR in COVID-19 diagnosis. It was observed that both 3-sample and 5-sample pooling are almost equally accurate and had a perfect agreement with individual sample testing. However, 3-sample pooling showed low false negativity and had high concordance rates with individual sample testing. The majority of people remain asymptomatic even after the infection of SARS-CoV-2 [14]. Mass screening using pooled sample strategy may be implemented to find out positive cases to restrict the community transmission of SARS-CoV-2 [15]. Moreover, in previous studies, pooled sample testing is an effective strategy to save resources and increase the capacity of diagnostic laboratories for COVID-19 surveillance, especially in low-resource settings [16]. The present study found that batched testing of pooled samples significantly reduced the cost of testing. Similar findings had been observed in previous studies with those of current work, which releveled that pooling helps to manage the global scarcity of testing resources, thus increasing the cost-effectiveness without much hampering the accuracy and sensitivity of qRT-PCR in detecting SARS-CoV-2 [17, 18]. In this study, only two pool groups were evaluated regarding their sensitivity and cost-effectiveness. The marginal discordance of 5-sample pooling with single-sample testing focuses on the inadequacy of pooled sample testing in detecting the specimens with low positivity. In future research studies, sample pooling should be optimized by considering the range of Ct (cycle of threshold) value of samples to avoid false negativity in the low positive specimens [19] Moreover, Costa et al [20] investigated and found that qRT-PCR pool testing methods is a helpful surveillance technique to find new SARS-CoV-2 variants and assess the duration of immunogenicity and overall immunity from vaccinations the general population. The pooling technique is an intriguing tactic for the health crisis brought on by the COVID-19 pandemic, optimizes the number of tests carried out and decreases the delivery time of results. We can screen COVID-19 samples through pool testing to save material and labour resources (public health and medical laboratory personnel). With the pandemic's constantly rising case count, pool testing has a clear advantage in resource conservation [21, 22].
5. Conclusion
Based on this study, it can be concluded that all the strategies regarding SARS-CoV-2 pooling are worth to develop so the mass testing can be more cost-effective. Moreover, pooling strategies could also speed up patient screening during the infectious disease outbreak by maximizing the current testing capacity of detection in both community and healthcare workers. Based on the prevalence rate of COVID-19 in particular regions, laboratories must work on their pool validation studies for RNA extraction and amplification kits. Overall, pooling is a cost-effective approach to increase the mass screening capacity of laboratories for clinical samples through qRT-PCR. The number of tests to be performed in the mass screening of samples decreases significantly by implementing sample pooling, thus, generating less laboratory waste.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Ethical Approval
This study was approved by the Institutional Ethical Committee, Chhattisgarh Institute of Medical Sciences, Bilaspur, Chhattisgarh, India (Ref. No. 211/I.E.C./CIMS/2022).
Conflict Of Interest
The authors declare no conflict of interest.
Acknowledgement
The authors are thankful to the Dean, and all virology staff, at Chhattisgarh Institute of Medical Sciences, Bilaspur, Chhattisgarh, India, for their support. We also thank Chhattisgarh Medical Services Corporation (CGMSC), South Eastern Coal India Ltd. (SECL) and Azim Premji Foundation for providing essential equipment and chemicals.
References
- Organization WH, WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 (2020).
- Long C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 126 (2020): 108961.
- Wiersinga WJ, et al. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 324 (2020): 782-793.
- Abbasi-Oshaghi E, et al. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): Laboratory, PCR, and chest CT imaging findings. Int J Surg 79 (2020): 143-153.
- Krause PR, et al. SARS-CoV-2 Variants and Vaccines. N Engl J Med 385 (2021): 179-186.
- Carpenter RE, et al. Confirming Multiplex RT-qPCR Use in COVID-19 with Next-Generation Sequencing: Strategies for Epidemiological Advantage. Glob Health Epidemiol Genom (2022): 2270965.
- Mahmoud SA, et al. Evaluation of pooling of samples for testing SARS-CoV- 2 for mass screening of COVID-19. BMC Infect Dis 21 (2021): 360.
- Shen M, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal 10 (2020): 97-101.
- Sawicki R, et al. Sample pooling as a strategy for community monitoring for SARS-CoV-2. Sci Rep 11 (2021): 3122.
- Griesemer SB, G Van Slyke and K St George. Assessment of Sample Pooling for Clinical SARS-CoV-2 Testing. J Clin Microbiol 59 (2021).
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22 (2012): 276-82.
- Hofman P, et al. Evaluation of Sample Pooling for SARS-CoV-2 Detection in Nasopharyngeal Swab and Saliva Samples with the Idylla SARS-CoV-2 Test. Microbiol Spectr 9 (2021): e0099621.
- Reusken CBEM, et al. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Euro Surveill 25 (2020).
- Wang X, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis 94 (2020): 107-109.
- Dhama K, et al. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev 33 (2020).
- Joachim A, et al. Pooled RT-qPCR testing for SARS-CoV-2 surveillance in schools - a cluster randomised trial. EClinicalMedicine 39 (2021): 101082.
- Abdalhamid B, et al. Cost Effectiveness of Sample Pooling to Test for SARS-CoV-2. J Infect Dev Ctries 14 (2020): 1136-1137.
- Prakash S, et al. Feasibility, efficiency & effectiveness of pooled sample testing strategy (pooled NAAT) for molecular testing of COVID-19. Indian J Med Res 153 (2021): 227-232.
- More S, et al. Pooling of Nasopharyngeal Swab Samples to Overcome a Global Shortage of Real-Time Reverse Transcription-PCR COVID-19 Test Kits. J Clin Microbiol 59 (2021).
- Costa MS, et al. Detection of SARS-CoV-2 through pool testing for COVID-19: an integrative review. Rev Soc Bras Med Trop 54 (2021): e0276.
- Rohde R. COVID-19 Testing: Is it time to jump into the pool? (2020).
- Barathidasan R. et al. Pooled sample testing for COVID-19 diagnosis: Evaluation of bi-directional matrix pooling strategies. J Virol Methods 304 (2022): 114524.


 Impact Factor: * 5.814
Impact Factor: * 5.814 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 