Prediction of Severity of Acute Pancreatitis by Biochemical Markers: A Tertiary Care Hospital Study
Article Information
Shanjidah Hoque1, Rifat Hassan2, Naheen Rezuan Shehran Asif3
1Specialist, General Surgery, Evercare Hospital, Dhaka, Bangladesh
2Consultant, National ENT Institute, Tejgaon, Dhaka, Bangladesh
3MS ((Paediatric Surgery), Specialist, Evercare Hospital, Dhaka, Bangladesh
*Corresponding author: Shanjidah Hoque, Specialist, General Surgery, Evercare Hospital, Dhaka, Bangladesh
Received: 29 August 2023; Accepted: 06 September 2023; Published: 15 September 2023
Citation: Shanjidah Hoque, Rifat Hassan, Naheen Rezuan Shehran Asif. Prediction of Severity of Acute Pancreatitis by Biochemical Markers: A Tertiary Care Hospital Study. Fortune Journal of Health Sciences. 6 (2023): 319 - 324.
View / Download Pdf Share at FacebookAbstract
Background: Acute pancreatitis (AP) is an acute inflammatory condition of the pancreas which may extend to local and distant extra pancreatic tissues. It is a life-threatening disease that has many causes, few effective treatments and numerous serious complications. Early diagnosis of acute pancreatitis and effective treatment can significantly reduce the mortality and morbidity of such cases.
Aim of the study: The aim of this study was to predict the severity of acute pancreatitis by biochemical markers.
Methods: This was a prospective observational study which was conducted on 35 admitted patients with diagnosis of acute pancreatitis at BIRDEM General Hospital, Dhaka, Bangladesh from October 2016 to April 2017. Complete blood count, serum amylase, serum lipase, C-reactive protein and serum procalcitonin values were observed. Data were collected from history, clinical findings and investigations. All data were collected, processed and analyzed by using MS Office and SPSS version 20.0 as per need.
Results: Among 35 patients, age range was 28-76 years. Number of male patients was 19 and number of female patients was 16. Among total patients, 21(60%) had mild attack and 14 (40%) patients had severe attack of acute pancreatitis. C- reactive protein (CRP) and procalcitonin (PCT) can differentiate between mild and severe acute pancreatitis. Among the parameters of the patients, P value of CRP was 0.047 and P value of procalcitonin (PCT) was 0.032, less than 0.05 which were statistically significant. These studies have shown that, serum procalcitonin (PCT) is a good marker for predicting severity and development of organ failure in acute pancreatitis and it is superior to serum C- reactive protein. Total WBC count was high in both mild and severe acute group patients with pancreatitis patients. Haemoglobin values and other baseline parameters showed no significant differences between two groups of patients. Serum amylase and serum lipase values were high in both groups. The magnitude of the elevation of amylase and lipase does not predict disease severity.
Conclusion: C- reactive protein (CRP) and procalcitonin (PCT) can differentiate between mild and severe acute pancreatitis. This prospective study was undertaken to evaluate in 35 patients with acute pancreatitis whether the new marker of systemic inflammation, PCT, had predict severe acute pancreatitis. The result was compared with CRP. PCT had a sensitivity of 91% and a specificity of 81%, which was superior to other tests.
Keywords
Acute pancreatitis, Biochemical markers, Inflammation, Severity
Article Details
1. Introduction
The first description of the pancreas has been attributed to Herophilus of Chalkaidon about 300 B.C. The naming of this organ, pancreas (Greek: pan, all; kreas, flesh), was not recorded until 400 years later by Rufus of Ephesus (100 A.D.) [1]. The first classification system for acute pancreatitis was reported by Fitz in 1889 [2]. In 1901, Opie described the association of gallstones to acute pancreatitis [3]. Alcohol was firmly established as an important pathogenic factor in 1917 [4]. More than 100 years ago, Chiari (1896) proposed that, intra-pancreatic activation of zymogens leads to pancreatic auto-digestion and is a key factor in the pathogenesis of acute pancreatitis. The association of hyperamylasaemia with acute pancreatitis has been recognized since 1929. In the history of radiography, the pancreas was a hidden structure seen only indirectly through studies exploring the surrounding organs, such as barium examinations of the upper gastrointestinal tract. Sonography was the first method that permitted direct imaging of the pancreas [5]. Pancreatic imaging essentially developed further with the introduction of computed tomography (CT) [6]. The rationale for surgery in severe acute pancreatitis has evolved over the last 50 years. Initially, total pancreatectomy was often recommended but it resulted in very high mortality rates [7]. The current thinking is that, the patients with infected pancreatic necrosis benefit from surgical debridement and drainage of the infected and devitalized tissue [8]. Further, surgery is often necessary if aggressive organ support in an intensive care unit seems inadequate for an acute pancreatitis patient with organ dysfunction. Acute pancreatitis is a common emergency presentation, being responsible for 3% of all hospital admissions with acute abdominal pain in the UK [9]. The incidence rate of acute pancreatitis varies considerably in different countries. Low figures have been reported in England (10/100,000) and Germany (15/100,000) [10, 11]. In USA, acute pancreatitis affects around 40-80 per 100,000 of the general population [12]. In Finland, acute pancreatitis is a common disease and its incidence has been increasing from 47 to 73 per 100,000 inhabitants/year in 1970-1989 and the increase correlates with alcohol consumption [13]. The hospital admission rate for acute pancreatitis is 9.8 per year per 100 000 populations in the UK, although worldwide, the annual incidence may range from 5 to 50 per 100 000. The disease may occur at any age, with a peak in young men and older women [14].
2. Methodology
This was a prospective observational study, conducted on 35 admitted patients with diagnosis of acute pancreatitis at BIRDEM General Hospital, Dhaka, Bangladesh during the period from October 2016 to April 2017. All the selected patients were evaluated by taking detail history and physical examination. The whole intervention was conducted in accordance with the principles of human research specified in the Helsinki Declaration [15] and executed in compliance with currently applicable regulations and the provisions of the General Data Protection Regulation (GDPR) [16]. As per the inclusion criteria of this study, only diagnosed patients with acute pancreatitis were included. On the other hand, according to the exclusion criteria of this study, patients with acute pancreatitis with multiple comorbidities like cerebrovascular diseases, CKD etcetera were excluded. Investigations on CBC, serum amylase, serum lipase, C-reactive protein and procalcitonin had been done and level of that makers were evaluated to assess the severity of the acute pancreatitis. The study was approved by the ethical committee of the mentioned hospital. Proper written consents were taken from all the participants before data collection. All data were processed, analyzed and disseminated by using MS Excel and SPSS version 23 program as per necessity.
2.1 Inclusion criteria:
Patient were suffering from acute pancreatitis.
2.2 Exclusion criteria:
Patient were suffering from acute pancreatitis with multiple comorbidities like Cerebrovascular diseases, CKD etc.
3. Results
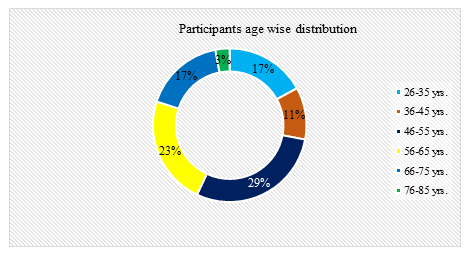
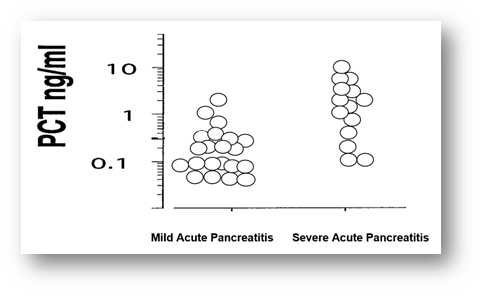
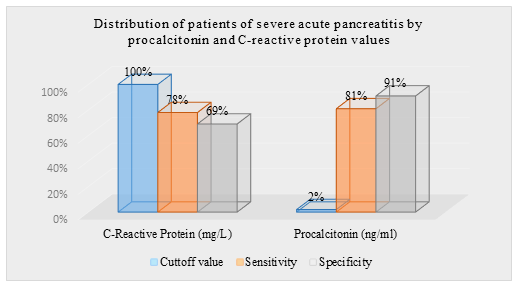
In this study, among total 35 participants, 54% were male whereas the rest 46% were female. So male participants were dominating in number and the male-female ratio was 1.2:1. Age distribution of all patients ranged from 28 to 76 years, where the youngest patient was of 28 years and the eldest was of 76 years. Among total patients, 10(29%) patients were within 46-55 years of age and 8(23%) patients were within 56-65 years of age. Among the parameters of the patients, P values of CRP was 0.047 and procalcitonin (PCT) was 0.032, less than 0.05 which were statistically significant. This study shown that, serum procalcitonin (PCT) is a good marker for predicting severity and development of organ failure in acute pancreatitis and it is superior to serum C reactive protein. Comparison between the two groups of patients showed that, the C-reactive protein (CRP) level was raised in 16 patients out of 21 patients with mild acute pancreatitis and 12 patients out of 14 patients with severe acute pancreatitis. About 80% patients showed raised C- reactive protein (CRP). Procalcitonin was significantly raised in 11 patients out of 14 patients with severe acute pancreatitis (range was 0.040-8.486) and also raised in 3 patients out of 21 patients with mild acute pancreatitis (range was 0.035-1.423). Total WBC count was high in both group of patients and markedly higher in severe group. Haemoglobin values showed no significant differences between two groups of patients. Serum amylase and serum lipase values were high in both groups. Study showed, serum amylase and serum lipase have significant p values for the diagnosis of acute pancreatitis but no role for the prediction of severity of acute pancreatitis. The magnitude of the elevation of amylase and lipase does not predict disease severity. The diagnostic accuracy of lipase appears to be better than that of amylase. Both serum amylase and serum lipase raised in 28(80%) patients with acute pancreatitis. Serum amylase and lipase was higher in severe acute pancreatitis (p< 0.05) but a high degree of overlap between values was found. The serum lipase values did not correlate with the severity of acute pancreatitis. Thus, both serum amylase and serum lipase were poor predictor of severity of acute pancreatitis. Complete blood count was done in all admitted patient with acute pancreatitis. Heamoglobin were more than 10g/dl in 11 patients out of 14 patients with severe acute pancreatitis and 18 patients out of 21 patients with mild acute pancreatitis. Total white blood cells count showed leucocytosis in 27 patients out of 35 patients. Total white blood count was markedly elevated in a group of patients with severe pancreatitis as range between 8.1-38.3 comparatively higher than mild group where the range was 6.04-22.3. Differential count showed elevated neutrophil count in 11 patients out 14 patients with severe acute pancreatitis. The ranges of the neutrophil count were 58-79% in mild group of patients and 62-82% in a group of patients with severe acute pancreatitis. Lymhocytopenia developed in 6 patients out of 14 patients with severe acute pancreatitis shown. The range of lymphocyte count was 12-41% in patients with mild acute pancreatitis and 17-37% in a group of patients with severe acute pancreatitis. Monocyte, eosinophil and basophil count were found within the normal limit in both groups of patients. The baseline characteristics showed no significant difference between the two groups. C - reactive protein (CRP) were higher in patients with severe acute pancreatitis than in those with mild acute pancreatitis. Circulating levels of C-reactive protein with mild acute pancreatitis (range was 3.7-101) and severe acute pancreatitis (Range was 6.0-113.6). The dotted line denotes median value. P-values is less than 0.05 which denote significance of difference between two groups. C-reactive protein has been showed to be a good severity predictor with a cut off level of 100 mg/l is used for distinguishing between the mild and the severe disease. Comparison with the C-reactive protein (CRP), procalcitonin (PCT) test had a sensitivity of 91% and specificity of 81% in severe acute pancreatitis. CRP was raised in 28 patients out of 35 patients and the rest of patients had raised (PCT) in 11 out of 35 patients. median value of CRP was 47.6mg/L and PCT was 0.07ng/ml. The ranges of CRP in total patients between 3.7-113.mg/L and ranges of PCT found between 0.035-8.4ng/ml. In this study, the sensitivity of serum amylase was found as 62% and specificity was found as 42% with the cut of value of 400U/L. Positive predictive value was 45% and negative predictive value of serum amylase was 88%. With the cut-off of 240 U/l, the specificity of serum lipase to detect a patient with acute pancreatitis was 88%, but the sensitivity was 79%. Serum lipase determination is recommended as a confirmatory test. But lipase has poor predictive value. The negative predictive values and positive predictive value for the procalcitonin were higher (90% and 53% respectively) than the respective values for CRP (89% and 47% respectively). The values of PCT showed high sensitivity of 91% and specificity of 81% in predicting severe acute pancreatitis. The negative predictive value was high (90%) indicating that with a negative test result severe acute pancreatitis can be excluded with a high probability.
Table 1: Results of Mann Whitney U test obtained by comparing parameters between mild and severe acute patient groups. (N=35)
|
Parameters |
Patients Group |
Frequency |
Median |
Range |
P value |
|
(n) |
|||||
|
Haemoglobin (g/dl) |
Mild |
18 |
10.1 |
8-13.9 |
0.0735 |
|
Severe |
11 |
11.4 |
8.9-16.6 |
||
|
Total WBC Count (x 109/L) |
Mild |
16 |
14 |
6.04-22.3 |
0.1002 |
|
Severe |
11 |
21.6 |
8.1-38.2 |
||
|
Serum Amylase (U/L) |
Mild |
9 |
617 |
34-1223 |
0.0916 |
|
Severe |
10 |
825 |
34-1480 |
||
|
Serum Lipase (U/L) |
Mild |
27 |
678 |
13-1463 |
0.0016 |
|
Severe |
5 |
1219 |
618-1613 |
||
|
C-reactive Protein (CRP) (mg/L) |
Mild |
16 |
47.6 |
3.7-101 |
0.0474 |
|
Severe |
12 |
78.7 |
6.0-113.6 |
||
|
Procalcitonin (PCT) (ng/ml) |
Mild |
3 |
0.062 |
0.035-1.423 |
0.0323 |
|
Severe |
11 |
0.386 |
0.040-8.486 |
Table 2: Distribution of study population by complete blood count. (N=35)
|
Parameter |
Patients |
Frequency |
Median |
Range |
Standard Deviation (SD) |
|
Group |
(n) |
||||
|
Haemoglobin (g/dl) |
Mild |
18 |
10.1 |
8-13.9 |
1.75 |
|
Severe |
11 |
11.4 |
8.9-16.6 |
||
|
White Blood Cells |
|||||
|
Total Count (/L) |
Mild |
16 |
14 |
6.04-22.3 |
10.97 |
|
Severe |
11 |
21.6 |
8.1-38.2 |
||
|
Differential count (%) |
|||||
|
Neutrophil |
Mild |
5 |
69 |
58-79 |
6 |
|
Severe |
11 |
75 |
62-82 |
||
|
Lymphocyte |
Mild |
15 |
29 |
Dec-41 |
8 |
|
Severe |
6 |
27 |
17-37 |
||
|
Monocyte |
Mild |
21 |
3.1 |
1.7-5.6 |
1.09 |
|
Severe |
14 |
3.1 |
1.8-6.6 |
||
|
Eosinophil |
Mild |
21 |
2.2 |
0.03-5 |
1.52 |
|
Severe |
14 |
2.2 |
0.05-6 |
||
|
Basophil |
Mild |
21 |
0.4 |
0.01-0.7 |
0.33 |
|
Severe |
14 |
0.4 |
0.03-0.9 |
||
Table 3: Distribution of patients of severe acute pancreatitis by procalcitonin and C-reactive protein values. (n=14)
|
Inflammatory Biomarkers |
Cutoff value |
Sensitivity |
Specificity |
|
C-Reactive Protein (mg/L) |
100 |
78 |
69 |
|
Procalcitonin (ng/ml) |
2 |
81 |
91 |
Table 4: Distribution of participants by serum procalcitonin and C-reactive protein values. (N=35)
|
Frequency (n) |
Median |
Range |
Standard Deviation (SD) |
|
|
C-Reactive Protein (mg/L) |
28 |
47.6 |
101(3.7-113.6) |
57.03 |
|
Procalcitonin (ng/ml) |
11 |
0.0761 |
8.451(0.035-8.486) |
2.13 |
Table 5: Comparison among the biochemical parameters. (N=35)
|
Variables |
Cut of value |
Sensitivity |
Specificity |
Positive predictive value |
Negative predictive value |
|
Serum Amylase |
400U/l |
62 |
42 |
45 |
88 |
|
Serum Lipase |
240 U/l |
79 |
88 |
58 |
91 |
|
CRP |
100 mg/L |
78 |
69 |
47 |
89 |
|
PCT |
2mg |
91 |
81 |
53 |
90 |
4. Discussion
Acute pancreatitis is a frequently seen disease with a wide clinical spectrum ranging from mild to severe. Most acute pancreatitis progress mildly and is self-limiting, however, 10–20% of the cases progress severely and 29-43% of severe cases progress fatally [17]. In our study we analyzed the role of complete blood counts, serum amylase, serum lipase, C- reactive protein and procalcitonin levels for the prediction of severity of acute pancreatitis in 35 patients. From the analysis and observation of the age distribution of patients showed that, all patients ranged from 28-76 years where the youngest patient was 28 years and the eldest patient was 76 years. Largest group of patients was aged between 46-55 years old that comprises 10 patients. The median age of the study population of 48 years appears significantly lower compared to another study in which the median age of acute pancreatitis was in the six decades [18]. All of our patients enrolled had predicted severe disease according to the widely used Atlanta classification system. A large number of studies have assessed the role of serum PCT and compared it to other inflammatory markers in predicting the severity of pancreatitis and the development of infected necrosis [19]. A prospective international malty center study by Bettina M et al [20] showed, lipase and amylase are both very specific laboratory tests for the diagnosis of acute pancreatitis when the suggested cut-off level is used. The more major imperfection is the lack of sensitivity of serum enzyme elevation to identify all those who have acute pancreatitis. Published experience has shown that acute pancreatitis is less likely to present with normal serum lipase than serum amylase values and this becomes more common with late presentations, when amylase levels tend to return to normal [21]. Serum lipase was elevated in 32 patients (91.42%) out of 35 patients and lipase was raised up to twenty-six times of its upper limit of normal range. Lipase has been shown to remain elevated longer than amylase after the onset of acute pancreatitis [22]. According to British Society of Gastroenterology guidelines for the management of acute pancreatitis, lipase is the main focus towards the diagnosis of acute pancreatitis [23]. CRP were higher in patients with severe acute pancreatitis than in those with mild acute pancreatitis. Levels of C - reactive protein with mild acute pancreatitis showed the range between 3.7-101 mg/L and severe acute pancreatitis where the range was 6.0-113.6mg/L. Serum C-reactive protein is frequently used for assessing the severity of acute pancreatitis. Opinions about its relevance in the early prediction of severe acute pancreatitis are divided. Tenner et al. reported that, CRP has no significance predictive role in assessing the severity of acute pancreatitis in the first 72 hours after admission [24]. A meta-analysis by Mofidi R et al. assessed the role of plasma procalcitonin in predicting the development of infected pancreatic necrosis and as well as the overall prognosis in non-infected severe acute pancreatitis. They concluded that plasma procalcitonin has a role in the management of both infected as well as non-infected severe acute pancreatitis [25]. CRP was elevated in 28 patients and serum procalcitonin was elevated in 11 patients out of 35 patients. Cardoso et al. in their study emphasized the role of CRP in the early assessment of the severity of acute pancreatitis, especially in the case of a mild form of the disease [26]. C-reactive protein is an easily detectable marker that is frequently used to predict the clinical severity of acute pancreatitis, necrosis and mortality. CRP is able to differentiate between mild and severe acute pancreatitis with high precision, and to predict the development of severe acute pancreatitis even at 24 hours following hospital admission [27]. Patients with CRP levels >150 mg/l on admission to the emergency unit and on transfer to the intensive care unit have been shown to have significantly and independently worse outcomes that those with lower CRP levels. Although there is a 24-48 hours’ latency period before CRP levels increase, which limits its utility as an early predictor of severity, CRP remains a useful predictor when levels have risen [28]. who reported higher sensitivity and specificity of serum lipase in diagnosis of acute pancreatitis compared to serum amylase. Early prediction of severity is an important goal in acute pancreatitis, in order to identify the 20% of patients who are likely to have a severe course. Such patients have an expected mortality of 15-20% and may benefit from early admission to high dependency or intensive care units [29].
Limitation of the Study
Time of acute attack and time of hospital admission may be the factor which may affect the values of the biochemical parameters. To know the usefulness of Procalcitonin (PCT) in acute pancreatitis, large prospective studies have to be conducted. This was a single centered study with small sized samples. Moreover, the study was conducted at a very short period of time. So, the findings of this study may not reflect the exact scenario of the whole country.
Conclusion & Recommendation
Determining individual values of CRP, PCT allows for the early detection of clinically severe forms of acute pancreatitis. Serum concentrations of amylase and lipase rise within hours of an episode of acute pancreatitis. They are key components of the diagnostic criteria along with acute abdominal pain. Serum lipase is now preferred over amylase due to a higher sensitivity. It also tends to remain elevated for longer than amylase, making it more useful when the presentation has been delayed by more than 24 hours. For the diagnosis of acute pancreatitis, serum amylase remains the most commonly used biochemical marker, but its sensitivity can be reduced by late presentation. Both enzymes may be elevated in various conditions other than pancreatitis. Neither is useful in monitoring the disease course or predicting severity. Serum lipase determination is recommended as a confirmatory test.
References
- Snezana Tesic Rajkovic et al. Prediction of acute pancreatitis severity via the combined analysis of inflammatory biomarkers and coagulation parameters 25 (2017): 238.
- Dina Zerem et al. Role of Clinical, Biochemical, and Imaging Parameters in predicting the Severity of Acute Pancreatitis 7 (2017): 1.
- William Steinberg, Scott Tenner. Acute pancreatitis 330 (1994): 1198.
- W R Matull et al. Biochemical markers of acute pancreatitis. J Clin Pathol 59 (2006): 340-344
- Chamara Basnayake, Dilip Ratnam. Blood tests for acute pancreatitis 38 (2015): 128-130.
- Åke Andrén Sandberg, Anders Borgström. Early Prediction of Severity in Acute Pancreatitis. Is This Possible? Pancreas (Online) 3 (2002): 116-125.
- Dina Zerem et al. Role of Clinical, Biochemical, and Imaging Parameters in predicting the Severity of Acute Pancreatitis 7 (2017): 2.
- Kylänpää?Bäck et al. Acute Pancreatitis: Diagnosis and assessment of severity with markers of inflammation 88 (2001): 222-227.
- Banerjee A, Kaul et al. Multicentre audit of death from acute pancreatitis. Br J Surg 81 (2004): 1541.
- Corfield A, Cooper M, Williamson R. Acute pancreatitis: a lethal disease of increasing incidence. Gut 26 (1985): 724-729.
- Assmus C Et al. Epidemiology of acute pancreatitis in a defined German population. Digestion 57 (1996): A217.
- Lankisch P Et al. Underestimation of acute pancreatitis: patients with only small increase in amylase/lipase levels can also have or develop severe acute pancreatitis. Gut 44 (1999): 542-544.
- Jaakkola M, Nordback I. Pancreatitis in Finland between 1970 and 1989 Gut 34 (2003): 1255-1260.
- Norman S. Williams, Christopher J.K. Bulstrode & P. Ronan O’Connell. Baily and love’s short practice of surgery 26 (2013): 1127.
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bulletin of the World Health Organization 79 (2001): 373 - 374.
- Voigt, Paul, and Axel von dem Bussche. "Enforcement and fines under the GDPR." The EU General Data Protection Regulation (GDPR). Springer, Cham (2017): 201-217.
- Forsmark CE and Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology 132 (2007): 2022-2044.
- Francisco et al. Factors related to length of hospital admission in mild interstitial acute pancreatitis 10 (2013): 105.
- Mándi Y, Farkas et al. Diagnostic relevance of procalcitonin, IL-6, and sICAM-1 in the prediction of infected necrosis in acute pancreatitis. Int J Pancreatol 28 (2000): 41-49.
- Rau BM et.al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg 45 (2000): 745-754.
- Frank B, Gottlieb K. Amylase normal, lipase elevated: Is it pancreatitis? Am J Gastroenterol 2 (1999): 463-469.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? Aust N Z J Surg 6 (2005): 399- 404.
- UK working party on acute pancreatitis. UK guidelines for the management of acute pancreatitis Gut 54 (2005): iii1-9. Tenner S, Baillie J, DeWitt J, Swaroop Vege S. American college of gastroenterology guideline: management of acute pancreatitis 9 (2013): 1400-15.
- Tenner S, Baillie J, DeWitt J, Swaroop Vege S. American college of gastroenterology guideline: management of acute pancreatitis 9 (2013): 1400-15.
- Mofidi R, Suttie et al. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review 146 (2009): 72-81
- Cardoso FS, Ricardo et al. C-reactive Protein at 24 Hours after Hospital Admission May Have Relevant Prognostic Accuracy in Acute Pancreatitis: A Retrospective Cohort Study. GE Port J Gastroenterol 22 (2015): 198-203.
- Mayer AD, McMahon MJ, Bowen et al. C reactive protein: an aid to assessment and monitoring of acute pancreatitis. J Clin Pathol 37 (1984): 207-211.
- Makela JT, Eila et al. Computed tomography severity index and C-reactive protein values predicting mortality in emergency and intensive care units for patients with severe acute pancreatitis. Am J Surg 194 (2007): 30–34.
- Kylanpaa-Back et al. Procalcitonin strip test in the early detection of severe acute pancreatitis. Br J Surg 88 (2001): 222-7.


 Impact Factor: * 5.814
Impact Factor: * 5.814 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 